Introduction
Carbofuran (2,3-dihydro-2,2-dimethylbenzofuran- 7-yl methylcarbamate, CAS number 1563-66-2) is a broad-spectrum systemic insecticide, nematicide and acaricide with a low residual effect, used worldwide. In 2007, authorizations for products containing carbofuran in the EU were cancelled because of the impact on human health and the environment. The specific toxicodynamic of this carbamate is associated to the inhibition of the enzyme acetylcholinesterase (AChE) and the neuronal synapses in the central nervous system. Therefore, AChE has been used in various biomonitoring programs, considered as one of the best indicators of effect on either acute or chronic exposure to piscine species (Li et al., 2011; Chen et al., 2012).
Disturbance in the oxidation/anti-oxidation balance is one of the main changes caused by carbamate pesticides (Abdollahi et al., 2004). This disturbance is called oxidative stress and carbamates induce it by catalyzing the conversion of oxygen to reactive oxygen species (ROS), which accumulate and overcome cellular and tissue antioxidant defenses. Lipid peroxidation (LPO) is a marker of effect, which reflects the action of ROS in biological lipids, and can be measured by the presence of a lipid metabolite, being malondialdehyde (MDA) the most representative decomposition product of lipoperoxides (Livingstone, 2001). Similarly, reduced glutathione (GSH) has several important functions, including protection against oxidative stress metabolites (Kanno et al., 2003). Fish respond to environmental stress through a variety of compensatory mechanisms, adapting their metabolic functions (Eissa et al., 2003), thus being good indicators of environmental quality. A possible example would be the tench, Tinca tinca L., which may constitute an adequate organism for biomonitoring in Southern Europe, as it is widely distributed in most small bodies of water, acting as bioindicators of regional environmental pollution in the whole water column due to their special behavior (Hernández-Moreno et al., 2008).
Brain has been reported as the most sensitive organ to an oxidative damage (Song et al., 2006), as well as the main reservoir for acetylcholinesterase. In the present study, the effect of different sublethal concentrations of carbofuran in brain of tenchs exposed under semi-static flow conditions for 28 days (long-term toxicity) was assessed. The effect after 7, 14 and 21 days of exposure was also studied. The objective was to determine the variations caused by such exposure in different biochemical parameters (oxidative stress and cholinesterase activity) of tench, in an attempt to establish parameters of early detection of pollution in a static polluted environment.
Materials and Methods
Ethical considerations
All procedures were performed in compliance with relevant laws and institutional guidelines concerning experimental animals.
Chemicals
All the chemicals and reagents (analytical grade) used were supplied by Sigma-Aldrich (Germany). Technical carbofuran (CF) (95% product) was provided by Faesal-Spain. Stock solution was prepared by dissolving pesticide in 1 mL of absolute ethanol in a standard volumetric flask. The amount of solvent in the aquaria was negligible.
Animals
Tenchs of both genders (mean weight 20±11 g) were obtained from a local fish hatchery (“Piscifactorías del Guadiana” in Badajoz, Spain). Animals (N=140) were transported in aerated water to the Toxicology Unit (Extremadura University, Spain) and randomly divided into four groups of 35 fish. Every group was placed into an isolated glass aquarium fitted with recirculated, dechlorinated and aerated tap water at 20±2 ºC. All animals were acclimated to laboratory conditions for 14 days prior the initiation of the study to reduce differences associated to stress, and were fed once daily with a commercial dry diet (OVN Dibaq-Diproteg, Segovia, Spain) until the end of the experiment. Water quality characteristics during all the experience were determined according to APHA (APHA/AWWA/WPCF, 1992). A 10-hours light controlled photoperiod was maintained.
Exposure
As indicated, fish were divided into 4 groups: one control and three experimental treatments. Each experimental group was exposed to a different sub- lethal concentration of pesticide (50, 100 and 200 μg/L) for 28 days, and more specifically, the lowest concentration used in this experiment corresponded to the concentration recommended in different countries for use in crop cultures (Clasen et al., 2014). Water in each aquarium was gently siphoned out twice a week and replaced with the same concentrations of pesticide to clean the water and preserve the pesticide concentration stable.
Water samples (250 mL) were taken once a week from each tank for carbofuran analysis to assess the effective concentrations of the pesticide by using a HPLC technique with acetonitrile and water as mobile phase and a Nucleosyl 120 C18 column (Hernández- Moreno et al., 2008). Calibrations were performed analyzing several samples of carbofuran at known concentrations. Carbofuran was also analyzed in muscle samples. Exposed and control animals (n=7 from each aquarium) were removed at the beginning of the experiment and at 7, 14, 21 and 28 days. A number of animals greater than 4 was taken from every aquarium at every sampling day, in agreement with the proposal for bioconcentration tests in continuous flow conditions (OECD, 1996), and each animal was considered a replicate. Fish were starved for 24 h before sacrifice.
Fish were anesthetized with a solution of MS222 (Sigma) at a concentration of 0.1 g/L (Hernández- Moreno et al., 2008). Animals were killed, and their brain was immediately dissected, weighed, measured and individually frozen at -80 °C until biochemical assays. Liver was taken to evaluate hepatosomatic index.
Biochemical measurements
Brain was homogenized in cold potassium phosphate buffer (100 mM, pH 7.4) at a concentration of 1 g/5mL. The homogenate was centrifuged at 3,000 rpm for 5 min at 4 °C to obtain the supernatant, where AChE activity was measured. An aliquot of brain homogenate was used to determine the extent of endogenous LPO and GSH levels after treatment with perchloric acid and a second centrifugation (4,000 rpm for 15 min at 4 °C).
AChE activity was determined spectrophotometrically at 405 nm, 42 ºC, according to the Ellman method (Ellman et al., 1961), adapted to a microplate, following the general procedure described by Thompson (1999), and expressed as Units (U) per mg of protein (1 U is equal to 1 nmol acetylcholine hydrolyzed per min). A FL600 microplate reader (Bio-Tek®, Winooski, VT, USA) was used to these purposes, since Tor et al. (1994) demonstrated the ability of a 96-well microplate reader for routine determinations of enzyme activity with sensitive, accurate, reproducible, and widely applicable results.
Accumulation of MDA was used as an index of lipid peroxidation and evaluated spectrophotometrically at 550 nm by measuring the presence of thiobarbituric acid reactive substances, according to the method proposed by Recknagel et al. (1982), using the microplate reader previously indicated. GSH levels were measured using a fluorometric method (Hissin and Hilf, 1976) at 350 nm excitation and 425 nm emission. In all cases, protein content in the homogenates was estimated by the Bradford method (Bradford, 1976) using bovine serum albumin as standard.
Statistical analyses
The SPSS software version 15.0 for Windows was used for data processing. Data were analyzed with one-way analysis of variance (ANOVA). In cases of significant differences, the ANOVA was followed by the LSD (Least Significant Difference) post-hoc test. Data were expressed as mean ± S.E. Significant limit was set up at p<0.05. A Pearson’s correlation test was also carried out.
Results
Water quality was analysed in order to maintain fish under controlled conditions. Temperature, pH, alkalinity, conductivity and hardness of aquatic media averaged 20-23.2 ºC, 7.8±0.5 (Crison pHmeter, Crison Instruments, Barcelona, Spain), 3.5±0.64 mmol/L, 0.52-0.76 mOhm and 160±41 mg/L (as CaCO3), respectively, and dissolved oxygen content was never below 5 mg/L. Fish were weighed and sized; obtaining means (± S.E.) of 65.04±7.81 g and 15.13±0.25 cm, respectively. Statistical analysis demonstrated a lack of significant difference among fish related to these biometric parameters (Table 1).
Table 1 Mean value ± SEM for the hepatosomatic index (hsi), weight (g) and length (cm) of tenchs sampled at the beginning of the experiment and at 7, 14, 21 and 28 days of exposure.
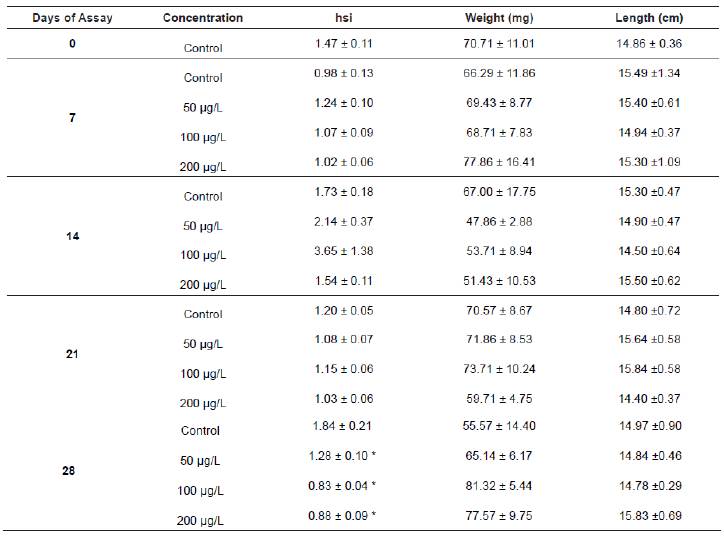
* Statistically significant when compared to the control (p<0.05).
The hepatosomatic index was altered after the last day of exposure to all the carbofuran concentrations, being statistically decreased (p<0.05) compared to the control. Since no changes were observed during the rest of the experiment (Table 1), it seems that the normal hepatopancreas development was only affected after a long exposure to the pesticide.
According to the conditions established by the OCDE guidelines for testing of chemicals with fish, the bioassay performed with tench exposed to carbofuran was considered valid, since no mortality was observed in the control group; the variation of pH values was always lower than 1 pH unity. Moreover, there was neither mortality nor nervous symptoms in any of the fish groups for the duration of the experiment.
Carbofuran residues were monitored in the water of the laboratory tanks to verify the presence of the active ingredient in the water. The test of water samples showed values around 90% in the nominal concentrations, assuming that fish were exposed to desired concentrations of carbofuran, during the whole experiment.
With respect to muscle samples, they were processed with dichloromethane and purified through a gel column to obtain extracts for injection in GC-MS. As an example, Figure 1 shows the profiles after injection of a sample from a tench exposed to 100 µg/L of carbofuran, with a clear peak appearing after 14.787 min easily related to carbofuran when observing the mass spectrum (Figure 2). Therefore, presence of carbofuran into the fish body was confirmed.
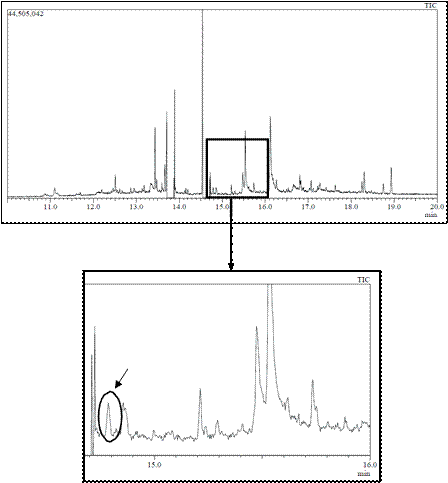
Figure 1 GC-MS chromatogram from muscle sample of tench exposed to 100 µg/L of carbofuran. The circle shows the carbofuran peak, appeared after a retention time of 14.787 min.
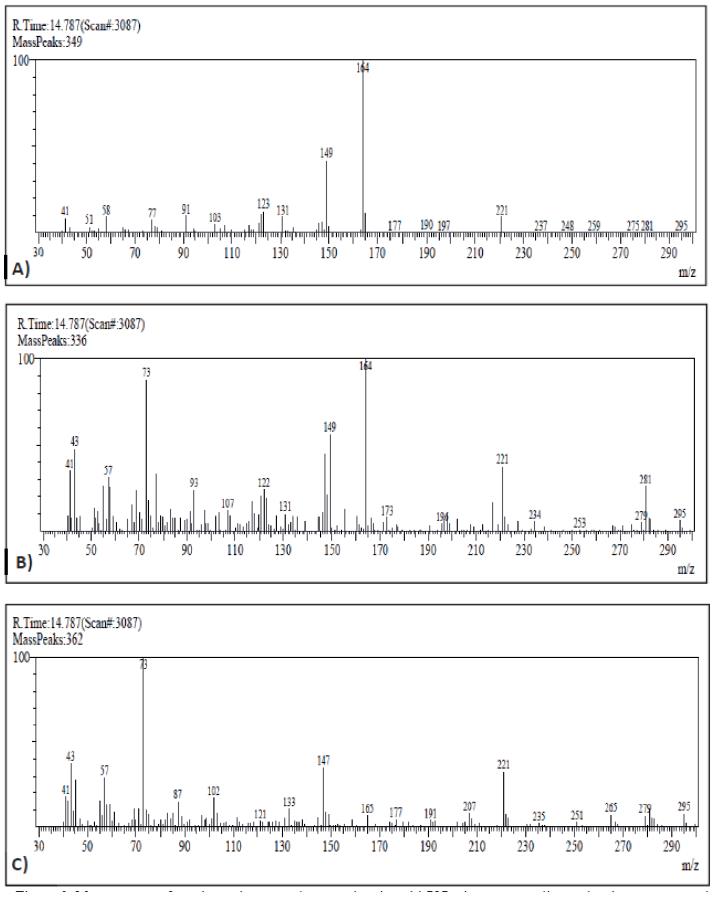
Figure 2 Mass spectrum from the peak appeared at retention time: 14.787 min, corresponding to the chromatograms obtained of carbofuran standard (A), muscle from fish exposed to 100 µg/L of carbofuran (B), and muscle from control fish (C).
Figure 3A shows LPO levels, measured as MDA concentration, in the brain of pesticide-exposed and control groups, expressed as % of control values. MDA levels were similar among groups until day 14. The MDA concentration was significantly decreased (p<0.05) in fish exposed to 100 μg/L CF after 14 and 28 days of the experiment (52.87 and 48.82% of decrease, respectively).
Afterwards, with the highest concentration (200 μg/L), there were three decreases in MDA content of 48.36, 61.78, and 62.97% at 14, 21, and 28 days of experiment, respectively, in all cases statistically significant (p<0.05). Moreover, animals exposed to 100 μg/L also showed a transient significant induction of MDA (p<0.05) 7 days after the beginning of the exposure (in that case related to an increase of 63.47%, when compared to the control).
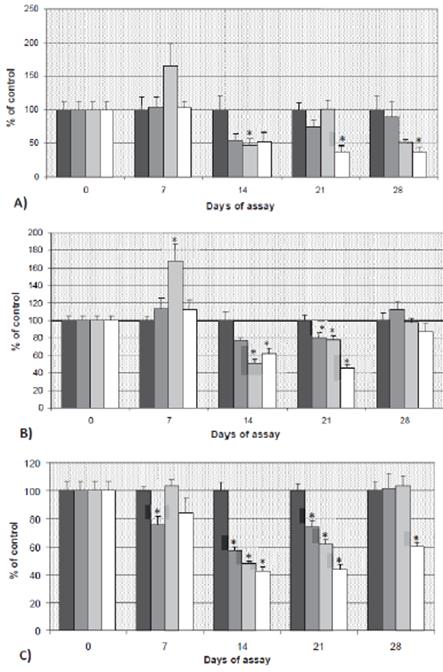
Figure 3 A) MDA, B) GSH and C) AChE levels in brain (mean ± SE) of tench exposed to carbofuran, expressed as percentage of control (100 %). *: statistically significant with respect to the control at the same sampling day (p<0.05). C1: 50 μg/L; C2: 100 μg/L and C3: 200 μg/L of carbofuran.
Regarding non-enzymatic antioxidant mechanism (GSH), increased (p<0.05) values were observed in fish exposed to 100 μg/L at 7 days of exposure when compared to control fish (Figure 3B). However, after 14 and 21 days those differences corresponded to decreased values of GSH. At 14 days of experiment, GSH levels decreased to 49.13 and 37.72% when compared to controls, with concentrations of 100 and 200 μg/L, respectively (p<0.05). Similarly, after 21 days, GSH levels decreased to 21.81 and 53.95% of control, for these two same doses of 100 and 200 μg/L, respectively. Nevertheless, and unlike that observed to MDA, GSH recovered to control levels after 28 days of exposure.
AChE activity results are shown in Figure 3C. Differences with the control group were observed since the beginning of the experiment, with the inhibition of 24.16% in fish exposed to 50 μg/L for 7 days. Fish exposed to all the concentrations, sampled at days 14 and 21, showed inhibited AChE activity (p<0.05). The highest inhibition occurred with 200 μg/L of carbofuran; this effect persisted until the end of the experiment. The inhibition caused by 50 and 100 μg/L was totally reverted at day 28 after exposure.
Regarding Pearson’s test, a positive correlation between AChE activity and MDA levels (r=0.345, p<0.05) was observed in control fish, being strongly significant the positive correlation found in fish exposed to 200 μg/L of CF (r=0.485, p<0.01). Regarding the relationship between MDA and GSH, a strong positive correlation appeared only in the control group (r=0.546, p<0.001), indicating possible disturbance in the oxidative stress complex after exposure to carbofuran.
Discussion
Tenchs showed decreased HIS at the end of the exposure period with all the tested concentrations. This effect was also found by Ram and Singh (1988) in Channa punctatus after 6 months of exposure to 996 mg/L of carbofuran. Those authors pointed out to a possible histopathological induction and biochemical alteration in liver that may cause physiometabolic dysfunction in this species, which can be extended to tench. As aquatic organisms have developed several cellular defense paths, which under normal metabolic conditions protect against the deleterious effects of chemical contaminants, lipid peroxidation and GSH levels are directly related to oxidative stress enzymatic activities. Variation in those biochemical parameters may result from stress situations, but susceptibility of the species should be also taken into account. The increase in xenobiotic concentration may not be always related to an induction of antioxidant activity since several factors can influence this effect (Hernández-Moreno et al., 2008). That idea is in accordance with the different effect on GSH levels showed by our tenchs exposed to 100 μg/L, increased at 7 days and decreased after 14 and 21 days of experiment.
Increased GSH levels observed in controls can be due to several exogenous factors more than exposure to pesticides, i.e. change of habitat (Sarkar et al., 2006). In this sense, a comparison of every exposed group with the control one is of great importance to identify the real effect of xenobiotics, since physiological levels can be modified along the time.
Several studies mentioned GSH levels as a useful tool for oxidative stress evaluation in fish, and more specifically cytotoxicity of some carbamates is correlated with the decrease in GSH content (Hernández-Moreno et al., 2010). An increase of glutathione levels was reported in catfish (Ictalurus punctatus) and marine fish exposed to sediments containing polycyclic aromatic hydrocarbon (PAH) (Di Giulio et al., 1993). Generally, antioxidant system induction indicates an adaptability response, thus allowing to overcome oxidative stress due to chemical exposure. Doyotte et al. (1997) and Zhang et al. (2004) reported increased GSH levels during a moderate oxidative stress process as an adaptation mechanism through the increase in its synthesis. Additionally, other biochemical responses can be seen after exposure to environmental contaminants. For example, suppression of GSH due to loss of adaptation mechanism and oxidation to GSSG after exposure to xenobiotics was reported by Zhang et al. (2004). In our study, a decreased GSH appeared after 14 days of the experiment; and similarly Ghosh et al. (1993) reported a time-related decrease in GSH levels in fish exposed to carbaryl, indicating that non-lethal concentrations are higher enough to deplete GSH.
Some authors consider GSH depletion as the main cause for a reduction of the free radical scavenging capacity of cells (Sevgiler et al., 2007; Kavitha and Rao, 2009), with LPO (measured as MDA levels) appearing closely related to GSH reductions. It has been suggested that many pollutants such as pesticides are capable of inducing oxidative stress through overproduction of reactive oxygen species (ROS): oxidative stress causes damage to cellular macromolecules such as nucleic acids, proteins and lipids, and lipid peroxidation has been suggested to be one of the molecular mechanisms involved in the cytotoxicity of carbamates (Üner et al., 2009). In fact, a general increase of MDA levels is usually associated to carbamate exposure (Hernández- Moreno et al., 2008; Ghosh et al., 1993). However, a clear inhibition of brain MDA levels has been obtained in the present study, thus indicating a decrease in the rate of lipid peroxidation. This is not an unusual finding; similar decreased levels of LPO were found by Üner et al. (2009) with fish exposed to organophosphates, whereas Song et al. (2006) showed the brain as the most sensitive organ to oxidative damage. Similarly, several authors reported brain as extremely susceptible to oxidative stress due to its high polyunsaturated fatty acids content, which quickly suffer peroxidation linked to a low antioxidant capacity (Sharma et al., 2005, Ballesteros et al., 2009). But the exposure concentration of the assay can clearly influence the brain LPO, as some previous experiments with tench exposed to carbofuran have demonstrated (Hernández-Moreno et al., 2010), thus reflecting that higher carbofuran levels are needed to observe an effect on LPO. This idea is supported by the lack of effect in MDA levels observed in carps when they were exposed to carbofuran for 15 and 30 days on a continuous flow, at 100 μg/L as the highest concentration (Ensibi et al., 2014). Nevertheless, those authors reported an early decrease after only 4 days of exposure, with a following recovery of MDA levels. In our study, it seems that the already known inversely correlation between MDA and GSH levels only appears after a long term exposure to carbofuran.
Brain AChE sensitivity towards several inhibitors has been used as a biomarker to identify organophosphate and carbamate exposure since 1970 (Payne et al., 1996; Fulton and Key, 2001). However, this inhibition is not always reached; as reported by Fernández et al. (1996) in a study with gilt-head bream exposed to malathion, where no differences appeared between control and exposed fish. Nevertheless, inhibition of 50% due to organophosphates and carbamates would indicate toxicity (Dembélé et al., 2000).
Depending on the species, some fish can survive with high levels of AChE inhibition, whereas other species die at the same concentrations. In this sense, carps exposed to fenthion survived even with inhibition of 90% (Üner et al., 2009), as well as C. auratus and carps exposed to carbofuran (Bretaud et al., 2000; Clasen et al., 2014), with AChE inhibition of up to 87%. Nevertheless, 70-80% of AChE inhibition is generally assumed as cause of death (Bradbury et al., 2008). That variability may be due to absorption, as well as metabolic and physiological factors, or different biochemical properties of the enzymes. Moreover, recovery of basal levels is different depending of the anticholinergic chemicals; therefore, effects of pesticides can differ depending on several factors (Ferrari et al., 2004).
In agreement with our results, other authors have reported neurotoxic effects along the time associated to acute or repeated exposure to high concentrations of carbamates (Moreno-Grau, 2003). The AChE activity pattern is similar to that reported by Wang et al. (2009), who developed a study with C. auratus exposed to propoxur for 15 days, showing a recovery period. The AChE activity of our animals recovered to basal levels the last day of the experiment at the lowest concentrations assessed, something reported also in C. auratus (Ferrari et al., 2004). Barata et al. (2004) also found recovery of AChE levels in Daphnia magna, being more rapid the recovery after exposure to carbamates than organophosphates. Moreover, when the inhibition is due to carbamate exposure, the reactivation of the enzyme appears spontaneously in a short period of time, helped by the rapid synthesis and release of new enzyme from the liver (Blaber and Creasey, 1960; Thompson, 1999). This suggests that a chronic exposure to some chemicals may trigger a physiological adaptation in aquatic organisms, inducing compensatory mechanisms.
In the present study, fish recovered basal AChE levels with no need of being transferred to pesticide-free waters, possibly due to adaptation or higher enzymatic synthesis facing to long-term exposure to low concentrations of carbofuran, as reported in previous experiments with fish exposed to lower concentrations for more than 30 days (Hernández- Moreno et al., 2010). Therefore, the link between recovery potential and concentration and time after exposure to a pesticide is confirmed (Kristoff et al., 2006). Indeed, the toxicity of carbofuran is highly dependent on the length, frequency and intensity of exposure, as well as the susceptibility of the organism tested, the latter being influenced by age, gender and genetic variation (Lannacone and Tejada, 2007).
As expected, AChE activity decreased as pesticide concentration and time of exposure increased, according to the results reported by Feng et al. (2008), Cong et al. (2009), and Clasen et al. (2014) in experiments with organophosphates and carbamates. Bretaut et al. (2000) also reported the same pattern, showing recovery of AChE levels at 50 µg/L after 14 days of exposure. Recovery of AChE levels may be influenced by several exogenous factors (time, temperature, salinity), as reported by Gholami- Seyedkolaei et al. (2013); therefore, it is of great importance to maintain the experiment under controlled conditions.
The increase in the acetylcholine concentration due to inhibition of AChE by carbofuran, leads to hypercholinergic excitatory processes and increases the rate of oxygen flow through brain and muscle, followed by a high increase in ATP consumption, much higher than its rate of production. This metabolic alteration has been described as a cause for reactive oxygen species production (Rai et al., 2011; Jaiswal et al., 2013), showing the relationship between neurotoxic and oxidative stress biomarkers. In fact, other similar studies with low concentrations of carbofuran have detected disruption of normal metabolism in fish organs, rendering antioxidant responses as biomarkers of carbamate exposure, together with AChE in brain (Clasen et al., 2014).
Our results demonstrate that the pesticide carbofuran may cause changes in some biochemical parameters of tench under laboratory conditions. In fact, changes in the three parameters assessed (but mainly inhibition of AChE activity) could be used as biomarkers of short exposure (less than 14 days) to carbamates in biomonitoring programs, whereas MDA reduction could also be associated with longer exposure to low concentrations of pesticides. The measurement of such parameters in brain samples could be taken into consideration to monitor insecticide toxicity to fish living in contaminated waters.














