Introduction
Infectious bovine keratoconjunctivitis (IBK, also known as “pink eye”) is a disease distributed worldwide and mainly associated to Gram-negative coccobacillus bacterium Moraxella bovis; but other agents such as M. ovis, Mycoplasma bovoculi, and Clamydophila spp., have also been implicated (Alexander, 2010). In addition, Moraxella bovoculi has been considered as a potential causal organism (Angelos et al., 2007; Sosa and Zunino, 2013).
Corneal ulceration caused by IBK often heals without therapeutic intervention and cattle generally recover; however, corneal rupture can result in complete and permanent loss of vision in severe cases, with marked ocular discomfort (Williams, 2010). Besides welfare implications, IBK also has a considerable economic impact, particularly due to reduced weight gain in calves suffering from IBK at weaning, and the cost of antibiotics (Schnee et al., 2015; Kowalski et al., 2017). Some studies have estimated losses of 10 to 20 kg of BW per infected animal. In the United States, 10 million cattle have this disease (Hare et al., 2008), causing losses of more than 200 million dollars per year (Addison, 2011).
In México, IBK is considered an enzootic disease, and it is associated to environmental conditions and seasonal vectors in some geographical regions. In the North of Mexico, the intense solar radiation is thought to be the most important factor favoring the presence of IBK. In the Central region of the country, it is associated with the presence of flyes and dusty winds, whereas in the South it is attributed to vectors (Gasque, 2008). The impact of this pathology in animal production in Mexico is unknown (Infante et al., 2000; Zamora et al., 2010).
Studies about the eye bacterial microbiota of healthy or diseased bovines are scarce. In addition, identification of microorganisms associated with IBK is required to establish if the clinical presentation is caused by secondary colonization of the damaged eye or if such microorganisms participate in the pathogenesis (Spradbrow, 1967; Handool, 2013; Sosa and Zunino, 2013). There is no information on the causal agent of IBK in Mexico; however, animals with eye lesions suggestive of IBK are frequently observed.
Therefore, the objective of this study was to report the molecular characterization of the ocular bacterial microbiota and their relation with IBK in cattle from two dairy regions in Michoacán, Mexico.
Materials and Methods
The study was carried out from July to December of 2015 in the localities of Uruétaro (19º 48' North latitude and 101º 10' West longitude, 1,860 m.a.s.l.), and Villa Madero (19º 59' North latitude and 103º 01' West longitude, 2,000 m.a.s.l.) in the Morelia-Queréndaro Valley dairy region, and Marcos Castellanos (19º 59' North latitude and 103º 01' West longitude, 2,000 m.a.s.l.), Sahuayo (20º 03' North latitude and 102º 44' West longitude, 1,530 m.a.s.l.), and Emiliano Zapata (20º 01' North latitude and 102º 36' West longitude, 1,540 m.a.s.l.) in the Ciénega de Chapala dairy region in Michoacán, under a hot subhumid climate.
Sampling
Samples were collected from 17 bovines (2.23%) showing ocular lesion, presumptive of IBK, from a population of 761 cattle. The animals were located in five localities and distributed in 11 herds. The number of affected animals and the number of herds sampled by locality were seven in Sahuayo (5 herds), four in Marcos Castellanos (3 herds), two in Uruétaro (1 herd), two in Emiliano Zapata (1 herd), and two in Villa Madero (1 herd). All were family herds under intensive (Uruétaro and Emiliano Zapata; 18.18%), extensive (Villa Madero; 9.09%), and semi-extensive systems (Sahuayo and Marcos Castellanos; 72.72%).
The animals were placed in a cattle chute designed to minimize stress during eye inspection and sampling, and were observed for uni- or bilateral ocular symptoms suggestive of IBK. Before sampling, the periocular region of the eye was cleaned with a gauze soaked into a 10% benzalkonium chloride soap, and 0.9% sodium chloride solution. Samples were taken from the ventral area of the eye, between the ocular globe and the conjunctival sac, using a sterile cotton swab, and then kept in a tube with Cary-Blair sterile medium (Copan Italia SpA, Brescia, Italy) until processing.
Culture of ocular samples
Culture was conducted within a laminar flow hood. The collected conjunctival swab samples were streaked on blood agar plates and grown 24 h at 37 °C. After incubation, colonies were observed under a microscope. The size, shape, edge, area, color, and presence of hemolysis in the colonies was evaluated. The gray-whitish, round, small convex colonies, with or without a hemolysis halo that could be associated with Moraxella were selected. The isolates were infused into a 15 ml Falcon tube containing 2.5 ml of Luria Bertani (LB) broth and incubated per 24 h at 37 °C under continuous stirring. One aliquot was mixed with 10% glycerol and stored at -80 oC. The remaining sample was used to carry out the Gram-staining, antimicrobial testing, and DNA extraction.
DNA extraction
Samples of 1.5 ml culture from bacterial isolates were grown overnight in LB broth. The suspension was used for DNA extraction by CTAB (hexadecyltrimethylammonium bromide) protocol (Minas et al., 2011). DNA was resuspended in deionized water and DNA integrity was verified by standard electrophoresis in 1% agarose gels.
Identification of bacterial isolates
In order to identify the bacterial isolates, a 1.5 kb fragment of the 16S ribosomal gene was amplified by PCR. Forward 5̒-AGAGTTTGATCCTGGCTGAG-3̒ and reverse 5̒-GGTTCCTTGTTACGACTT-3̒ oligonucleotides (Elim Biopharmaceuticals,Inc, Hayward, CA, USA) were used. PCR amplification was carried out using 50 ng of DNA and the Platinum PCR SuperMix High Fidelity (Invitrogen, California, USA) in a final volume of 20 µl. The same mix was used without DNA as a negative control. The amplification reaction was performed under the following conditions: an initial step at 95 °C for 5 min, and then 30 cycles of the program, 30 s at 95 °C for DNA denaturalization, 30 s at 58 °C for oligonucleotides alignment, and an extension at 72 °C for 1.5 min. At the end of the final amplification, a one extension at 72 °C for 5 min was performed. The integrity of the PCR products was revised and analyzed by electrophoresis in 1% agarose gels.
The PCR products were sequenced by Sanger technique by Elim Biopharmaceuticals, Inc (Hayward, CA, USA). The electropherograms were analyzed using the Mega 7.0.7 (DNASTAR) program. The sequences obtained from the bacterial 16S gene were compared with those available in the NCBI data bank to identify the isolates using the BLAST option (https://www.ncbi.nlm.nih.gov/guide/sequence-analysis/).
Antimicrobials tests
All of the bacterial isolates were tested for antimicrobial susceptibility, which was determined using the disk diffusion method on Mueller-Hinton (MH) agar plates (Bioxon, Mexico). The following disks for Gram-negative bacteria (Gram Negatives II Bio-Rad) were used: amikacin, 30 µg; ampicillin, 10 µg; levofloxacin, 5 µg; cephalothin, 30 µg; cefotaxime, 30 µg; ceftriaxone, 30 µg; chloramphenicol 30 µg; gentamicin, 10 µg; netilmicin 30 µg; nitrofurantoin 300 µg; cefepime 30 µg; trimethoprim-sulfamethoxazole 25 µg. In addition, the following antimicrobials used against Gram-positive bacteria were evaluated (Gram-positive, Bio-Rad, México): ceftazidime, 30 µg; cefuroxime, 30 µg; dicloxacillin, 1 µg; erythromycin, 15 µg; pefloxacin, 5 µg; penicillin, 10 U; tetracycline, 30 µg. Isolates were classified as susceptible, intermediate and resistant according to the manufacturer’s instructions.
An MH agar plate without antimicrobials was used as a control treatment. Plates were incubated at 37 ºC for 24 h.
Results
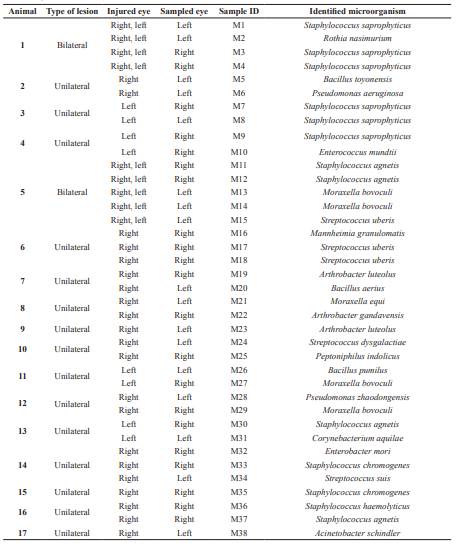
Seven hundred and sixty-one bovines from two dairy regions in Michoacán (México) were analyzed. According to symptoms, 17 animals (2.23%) showed IBK, mainly localized in one eye. Fifteen bovines showed unilateral lesions and only two showed lesions in both eyes. Based on the colony morphology, 38 colonies were isolated, of which 13 colonies were from samples of clinically healthy eyes, and 25 from cattle with morphological lesions (Table 1).
In a first approach, the bacterial isolates were identified using the Gram-staining. The results showed that 68.98% of the bacterial isolates were Gram-positive and 31.56% were Gram-negative. Furtherly, bacterial isolates were identified using the sequences of the 16S ribosomal RNA. In the Gram-positive samples, the most abundant bacterial microbiota corresponded to Staphylococcus saprophyticus (15.78%), Staphylococcus agnetis (10.25%), Streptococcus uberis (7.89%), Staphylococcus chromogenes, and Arthrobacter luteolus (5.26%). Staphylococcus haemolyticus, Streptococcus dysgalactiae, Streptococcus suis, Enterococcus mundtii, Bacillus aerius, Bacillus toyonensis, Bacillus pumilus, Rothia nasimurium, Arthrobacter gandavensis, Peptoniphilus indolicus, and Corynebacterium aquilae were present in 2.63%, each species. In relation to the Gram-negative isolates, the most abundant species was Moraxella bovoculi (10.52%), whereas the remaining identified microorganisms (Pseudomonas aeruginosa, Pseudomonas zhaodongensis, Mannheimia granulomatis, Acinetobacter schindler, Enterobacter mori, and Moraxella equi) showed frequencies of 2.63% (Table 2).
Antimicrobial sensitivity of bacteria isolates and their resistance patterns are shown in Table 3. Multi-resistance was observed for different groups of antibiotics. The 88.8% of isolates was resistant to dicloxacillin, 77.7% to ceftazidime, 55% to penicillin, 22.2% to tetracycline and ampicillin, and only one isolate was resistant to erythromycin.
The highest resistance rate of Gram-positive isolates was toward doxycycline (75%; 18/24), ceftazidime and penicillin (54.1%; 13/24). Interestingly, Staphylococcus isolates showed resistance mainly to ceftazidime, dicloxacillin, and penicillin. In the same way, isolates of Streptococcus uberis showed 100% resistance to dicloxacillin. Regarding to the genus Arthrobacter, isolates showed 100% resistance to penicillin, 66.6% to pefloxacin, and 33.3% to doxycycline. In addition, A. gandavensis showed resistance to cephalothin, ceftazidime, erythromycin, ampicillin, and doxycycline.
Seven Gram-negative isolates were observed, of which only M. granulomatis showed sensitivity to all antimicrobials. The remaining isolates showed resistance to antimicrobials with different patterns (Table 3). Noteworthy, P. aeruginosa (case 2) showed resistance to nitrofurantoin, chloramphenicol, ceftriaxone, ampicillin, trimiteprim sulfamethoxazole, cefotaxime, cephalothin and cefepime. Also, P. zhaodongenisis (case 6) only showed resistance to ampicillin and levofloxacin. Finally, E. mori (case 14), A. schindler (case 17), M. equi (case 8) and M. bovoculi (cases 5 and 11) showed resistance to ampicillin and cephalothin.
Discussion
Bacterial isolates from injured eyes were diverse and mainly Gram-positive (68.98%), similar to other reports in bovines (Sosa and Zunino, 2013), and humans with conjunctivitis (Hernández and Quintero, 2003). Presence of Gram-positive bacteria could be attributed to its resistance to adverse and dry conditions because they contain a thick cell-wall, rich in peptidoglycan (Russell, 2003). For the Gram-negative bacteria, the predominant genus was Moraxella.
Table 1 Microorganisms obtained from ocular samples of bovines with presumptive infectious keratoconjunctivitis (IBK) in Michoacán, Mexico.
In addition, more bacterial isolates were obtained from injured eyes in comparison with healthy eyes. This could be explained by the fact that defense mechanisms are affected in the injured cornea favoring the invasion of opportunist infectious agents.
One of the main predisposing factors for the presentation of IBK is the environment.Aprevious study by Takele and Zerihun (2000) in South-east Ethiopia showed an incidence of 2.10% IBK in local zebu and crossbreed dairy animals, which is similar to what was observed here. In that study, the researchers reported unilateral presentation in 85.5% of the cases, whereas bilateral infection was 14.5%. In our study 88.2 and 11.76% of unilateral and bilateral afections were observed, respectively. Aditonally, in 80% of reported IBK cases M. bovis has been isolated together with other bacteria such as Actinomyces piogenes, Staphylococcus aureus, Pasteurella haemolytica, Escherichia coli, and Proteus spp (Takele and Zerihun, 2000). In our study, the ocular bacterial microbiota was diverse, probably related with the environment and production system of each farm (intensive or semi-intensive), which may favor dissemination or growth of different bacteria populations. It is important to highlight that in this study, although we found presumptive symptomatology to IBK, this was associated with the presence of M. bovoculi and not to M. bovis as reported by Takele and Zerihun (2000). According to the above comments, it is necessary to conduct studies in Mexico's tropical areas to determine if M. bovis is the causal agent of IBK.
Studies in cattle where bacterial microbiota was identified show some of the species of bacteria reported here; i.e., Acinetobacter spp. (Wilcox, 1970; Hare et al., 2008; Sosa and Zunino, 2013), Bacillus spp., Corynebacterium (Spradbrow, 1967; Wilcox, 1970), Streptococcus ssp. (Sosa and Zunino, 2013), A. gandavensis, A. luteolus, Pseudomonas spp. (Hare et al., 2008, Sosa and Zunino, 2013), Arthrobacter (Sosa and Zunino, 2013), and M. bovoculi (Blood and Radostits, 1992; Angelos et al., 2007; Libardoni et al., 2007). Differences between studies could be attributed to geographical locations, which are expected to have different environmental conditions. In the same way, some of the bacteria isolated in this study have been associated with etiological agents of bovine conjunctivitis and bovine keratosis. However, other opportunistic bacteria living in the skin and nasal cavities are commonly found in the conjunctivae of the eyes of healthy animals (Handool, 2013), favored by farm environmental and management conditions.
Table 2 Frequency of bacterial isolates associated to presumptive infectious bovine keratoconjunctivitis (IBK) in catle in Michoacán, México.
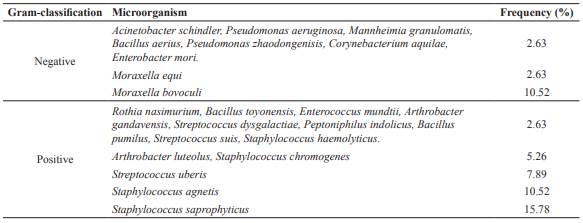
Table 3 Antimicrobial sensitivity of isolates associated with infectious bovine keroconjuntivitis (IBK) in catle in Michoacán, México.
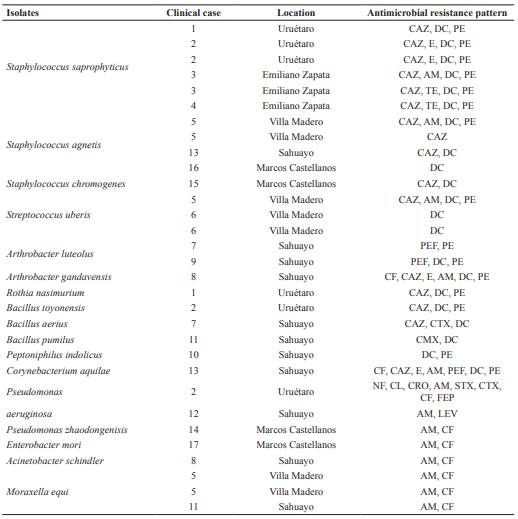
CF: Cephalothin 30 µg, CAZ: Ceftazidime 30 µg, E: Erythromycin 15 µg, AM: Ampicillin 10 µg, TE: Tetracycline 30 µg, STX: Trimethoprim sulfamethoxazole 25 µg, CTX: Cefotaxime 30 µg, GE: Gentamicin 10 µg, CMX: Cefuroxime 30 µg, PEF: Pefloxacin 30 µg, DC: Dicloxacillin 1 µg, PE: Penicillin 10U. NF: Nitrofurantoin 300 µg, CL: Chloramphenicol 30 µg, CRO: Ceftriaxone 30 µg, FEP: Cefepime 30 µg, LEV: Levofloxacin 5 µg.
Antimicrobial sensitivity tests showed that IBK-associated isolates possess extensive resistance to β-lactams, mainly penicillin, ampicillin, and doxycycline. Many Gram- negative bacteria have a naturally occurring chromosome-mediated β-lactamase that confers resistance to this group of antibiotics and the use of new β-lactams resistant to the hydrolytic action of β-lactamases has caused the emergence of new β-lactamases that favors resistance selection to those drugs (Bradford, 2001). Strains producing extended-spectrum beta-lactamases (ESBL), such as Gram-negative bacilli, mainly enterobacteria, are generally multi-resistant, especially beta- lactams. Bacterial resistance is also attributed to the common use of these drugs for the treatment of several infectious diseases in cattle (Ochoa et al., 2008). Presumably, the selective pressure derived from the use and abuse of new antibiotics has selected for new variants of β-lactamase. In this regard, multi-resistant isolates were observed. For example, isolates of the species A. gandavensis, C. aquilae and P. aeruginosa showed resistance to more than 50% of the tested antimicrobials. These resistance patterns are most often associated with the integration of new enzymes obtained by conjugation, transformation, or transduction (Navarro et al., 2010). Although this could explain the frequency of resistance observed to β-lactams in our study, molecular studies are needed to identify if they have this type of enzymes. Different resistance patterns may indicate the preferred use of antimicrobials to treat IBK in each region (Loy and Brodersen, 2014), and the bacterial microbiota associated with this pathology can be related to the frequency and pattern of use of antibiotics in dairy systems.
In conclusion, normal bacterial microbiota of the conjunctivae has been poorly studied, lacking phenotypic and genotypic indicators to compare the bacterial microbiota of the clinically healthy eye and animals with IBK. In this study, the bacterial isolates identified in eye lesions of cattle and associated to IBK was diverse. This is the first study on the subject conducted in Mexico. More studies on IBK are required under the conditions of Michoacán and other Mexican regions.














