Introduction
The spectacular growth performance experienced by broiler chickens over the last decades has been accompanied by metabolic disorders, low resistance to diseases, and increased deposition of body fat (Leeson and Zubair, 1997). This last condition in broiler chickens is inefficient in terms of energy metabolism and represents an economic loss for the producers (Tůmová and Teimouri, 2010). Growth rate and body composition throughout the lifecycle of animals are determined, among others, by feed consumption (Richards, 2003). The use of early nutrient-restriction programs has the potential to prevent complications such as increased body fat deposition and its consequences (i.e., reproductive failure, sudden death syndrome, cardiovascular disorders, impaired thermoregulation, and decreased immunity) (Sahraei, 2012; Zubair, 1996). Those feeding programs rely on the phenomenon called compensatory growth. Compensatory growth occurs when chickens whose growth has been retarded by dietary restriction grow faster than animals of the same age with no prior restriction (Lippens et al., 2000).
A wide range of alternatives have been used to induce this mechanism of growth regulation. Wilson and Osbourn (1960) concluded that compensatory growth after undernutrition was affected by the duration and timing of undernutrition, the nature and severity of undernutrition, genetic factors like sex, bird strain, and re-alimentation condition (Zubair, 1996). However, very few studies have considered the effect of nutrient concentration of the diets used during the refeeding period (Rahimi, 2015; Taschetto et al., 2012).
Feed restriction not always gives the expected results, and the best feed conversion does not guarantee the best economic efficiency (Jahanpour et al., 2015; Eila et al., 2011; Novele et al., 2008). Therefore, the present work aimed to understand the interaction between restriction level during the starter period and nutrient content of the diet in the growing and finishing periods. This study was conducted to assess the effect of two levels of feed restriction followed by a re-alimentation period with five increasing levels of nutrients on the growth performance and immune response of broiler chickens.
Materials and Methods
Ethical considerations
Use and care of birds and procedures in this study were approved by the Islamic Azad University Ethics Committee (protocol number 17.16.4.16987/2014).
Animals
Before starting the trial, the research facility was thoroughly cleaned and disinfected. Three hundred and thirty (one-day-old) male chickens of Ross 308 strain (Aviagen, Newbridge, UK) purchased from a commercial hatchery were used. The broiler chicks were placed in 1.5 × 1.0 m cages, which floor was covered with shredded paper. Each cage was equipped with a pan feeder and a manual drinker. The research facility was an open-sided poultry barn equipped with thermostatically controlled curtains, thermostatically controlled gasoline rocket heaters, overhead sprinklers, wall- mounted fans at both ends of the barn, and fluorescent tubes in ceiling fixtures. Ambient temperature was set at 32 °C for placement and then decreased gradually to achieve 24 °C from week 3 onwards. Lighting was constant on day 1. From day 2 to the end of the study, the light regime was 23L:1D.
The experiment lasted 42 days. The feeding program consisted of a starter diet until chicks were 14 days old, followed by a grower diet up to 28 days of age, and then a finisher diet until the end of the experiment. The diets were formulated according to a standard commercial program (Table 1).
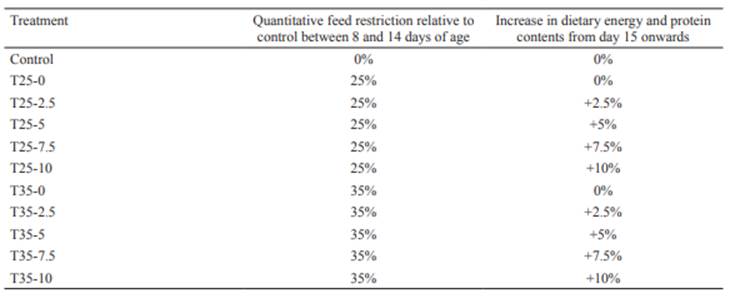
Chicks were randomly assigned into one of 11 treatments, including a control (Table 2), each of them having five replicates; thus there were a total of 55 groups of six birds.
Table 1 Experimental diets fed to broiler chickens.
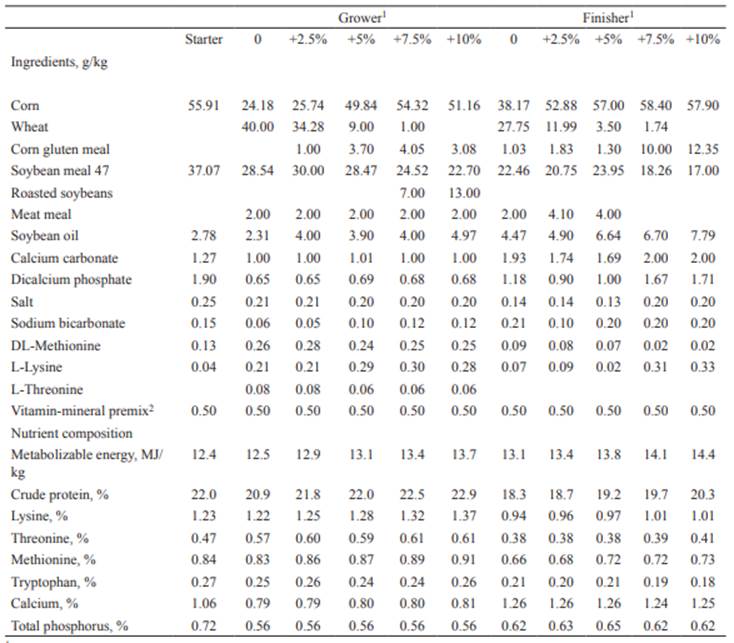
1 2.5, 5, 7.5, 10, and 15% increment of diet energy and protein concentrations over the control (0%).
2 Supplied per kilogram of feed - Vitamin A: 12,500 IU; vitamin D3: 1,250 IU; vitamin E: 18 IU; vitamin K3: 3.7 mg; thiamine: 1.8 mg; riboflavin: 6.6 mg; calcium pantothenate: 10 mg; niacin: 37.5 mg; pyridoxine: 32.5 mg; vitamin B12: 2.5 mg; Mn: 50 mg; Zn: 37.5 mg; Fe: 25 mg; Cu: 7.5 mg.
In treatments 2 to 11, feed restriction consisted of a daily feed supply adjustment between 8 and 14 days of age proportional to feed intake of control chicks in the previous day (75 and 65% in T25- and T35- treatments, respectively). In those treatments, more energy and protein content (T25-2.5, T25-5, T25-7.5, T25-10, T35-2.5, T35-5, T35-7.5, T35-10) or not (T25-0 and T35- 0) were supplied from day 15 onwards. Control broilers were fed ad libitum during the whole experimental period, while broilers in the other treatments were fed ad libitum before and after the feed restriction period. Body weight (BW) of the chicks and feed consumption were weekly recorded by cage, and body weight gain (BWG, g/period), feed intake (FI, g/period), and feed conversion ratio (FCR, feed to gain g/g) were determined. At the age of 42 days, after 4 hours of fasting for complete evacuation of the gut, five chickens per treatment (one from each replicate) that had weights closest to the mean weight for the cage were selected and euthanized by cervical dislocation (Leary et al., 2013). Birds were fully plucked by the dry plucking method, eviscerated, and weights of the organs related to the immune system were recorded.
Antibody production to different antigens was assessed during the experiment. First, the birds were vaccinated against influenza (1st day of age), infectious bronchitis (4th and 17th day of age), Newcastle disease (9th and 16th day of age), and Gumboro disease (14th and 23rd day of age). The vaccines were provided by Razi Co. (Tehran, Iran). Additionally, birds were injected under the breast skin with 0.5 ml of a 10% suspension in phosphate buffered saline of sheep red blood cells (SRBC) on the 22nd and 29th days of age. To determine the systemic antibody response, blood samples were collected from one chick per replicate via the wing vein on the 19th and 26th (Newcastle disease), 30th and 40th (influenza disease), and 29th and 36th (SRBC) days of age. Blood samples were processed and analyzed as described by Pourhossein et al. (2014). To determine antibody response to influenza and Newcastle disease, a hemagglutination inhibition assay was used. Total immunoglobulin (Ig) and immunoglobulin G (IgG) titers to SRBC were determined by hemagglutination assay; then, immunoglobulin M (IgM) titers to SRBC were calculated as the difference between total Ig and IgG titers.
Statistical analysis
The GLM procedure of SAS UE 3.5 (SAS Institute Inc., Cary, NC) was used for the analysis. The statistical model was Yijk = μ + Aj + Bk + ABjk + eijk, where Yij is the dependent variable; μ represents the overall mean; Aj is the fixed effect of restriction; Bk is the fixed effect of diet nutrient density after restriction cessation; ABjk is the interaction of both effects, and eijk is the residual error. The least square means in the treatments of the T25 and T35 groups were compared with those of the Control group by using Dunnet’s test. The linear and quadratic responses to refeeding levels within each feed restriction group were investigated through polynomial contrasts with the CONTRAST option. Correlations between the percentage of carcass fat and plasma lipids were investigated with the SAS CORR procedure. Statistical significance was declared at p<0.05.
Results
Mortality rate of broiler chickens was 0% in all the experimental treatments. In the results presented below, any significant differences at p<0.05 between the experimental treatments and the control treatment correspond to the superscripts A or B placed after the least squares mean in the tables. Growth performance traits during the different feeding periods are shown in Table 3.
As expected, there were no differences (p>0.05) in performance between treatments before the restriction period (1-7 d), but they were higher (p<0.05) in the control treatment during restriction. In the growing period, FI and BWG were higher, and FCR was lower (p<0.05) for all the T25 treatments, except for T25-0, compared with the control treatment, and the response of FI and BWG to increased nutrient density following feed restriction was mostly quadratic (p<0.001), whereas that of FCR was linear (p<0.001). The FCR results were lower in all the T35 treatments compared to the control (p<0.05); however, FI and BWG results were more heterogeneous in the T35 treatments. Contrary to the T25 group, the observed improvement in FCR in the T35 group was more clearly related to an increased BWG without a concomitant increase in FI. During finishing, no differences (p>0.05) were observed in FCR between T25 and T35 compared to the control treatment. When the whole experimental period was considered, BWG was higher and FCR was lower (p<0.05) in T25-5, T25-7.5 and T25-10 treatments compared to the control; those parameters showed an increasing and decreasing linear fashion (p<0.01), respectively, as nutrient density increased. The T35 group did not show clear responses. The only significant effect was the lower FI and FCR (p<0.05) in the T35-7.5 treatment compared to the control.
Very few effects and no clear lineal or quadratic responses were observed in the weight of immune-related organs as a percentage of BW, nor in the immune response to vaccines due to the treatments assayed (Table 4).
Discussion
Feed restriction programs in broilers are mainly intended to improve feed efficiency and decrease fat deposition without compromising the health status. Several reports have placed emphasis on quantitative feed restriction in the early stages of growth (Lippens et al., 2009; Mohebodini et al., 2009; Novele et al., 2009; Shabani et al., 2015) but few studies have investigated the effects of nutrient density of the diet after the feed restriction period (Giachetto et al., 2003; Leeson and Zubair, 1997; Rahimi et al., 2015; Santoso et al., 1995).
In the present study, the best FCR and highest BW at slaughter was observed in the T25 group compared to the control treatment, and the response was positively related to the nutrient density of the diet (Tables 2 and 4). Nevertheless, the T35 group showed full BW recovery at the end of the experimental period irrespective of nutrient density levels (Table 4), despite an 11% BW reduction at the end of the restriction period compared to the control. These results agreed with Rosa et al. (2000), who indicated that the occurrence of compensatory growth in broilers could be compromised when weight losses are over 11-12%. The occurrence of full BW recovery at slaughter age after quantitative feed restriction is contradictory (Jahanpour et al., 2015; Mohebodini et al., 2009; Saleh et al., 2005; Shabani et al., 2015), which might be explained by several factors, including severity, duration and timing of the restriction period, length of the refeeding period, and animal characteristics (Yu and Robinson, 1992), as well as physical form of the feed (Lippens et al., 2009; Shabani et al., 2015). On the other hand, our results support that full BW recovery does not seem always to be related to better FCR as reported by Butzen et al. (2013) and Novele et al. (2009). Our results are mostly in agreement with those of Rahimi et al. (2015), who tested 15 and 30% quantitative feed restriction between 8 and 14 days of age and higher energy and protein diets (5, 10, and 15%) in the feed restricted groups afterward. It is noteworthy that the average productive results of the T35 group in the present work were similar to those of the control treatment. In contrast, the T30 group of Rahimi et al. (2015) performed better than their control treatment. This point suggests a possible threshold in the quantitative feed restriction of broilers above which no improvement of productive results occurs. Moreover, in the T25 group, FCR improvements were only observed in the T25-5, T25-7.5, and T25-10 treatments, indicating that a minimum increase of dietary energy and protein density is needed during the refeeding period to achieve the best result.
Table 3 Feed intake (FI), body weight gain (BWG) and feed conversion rate (FCR) of broilers raised under a normal feeding program (control), or two levels of quantitative feed restriction (25 and 35%) between 8 and 14 days of age followed by refeeding energy and protein dense diets (0, 2.5, 5, 7.5 and 10% over the control diet) from 15 to 42 days of age.
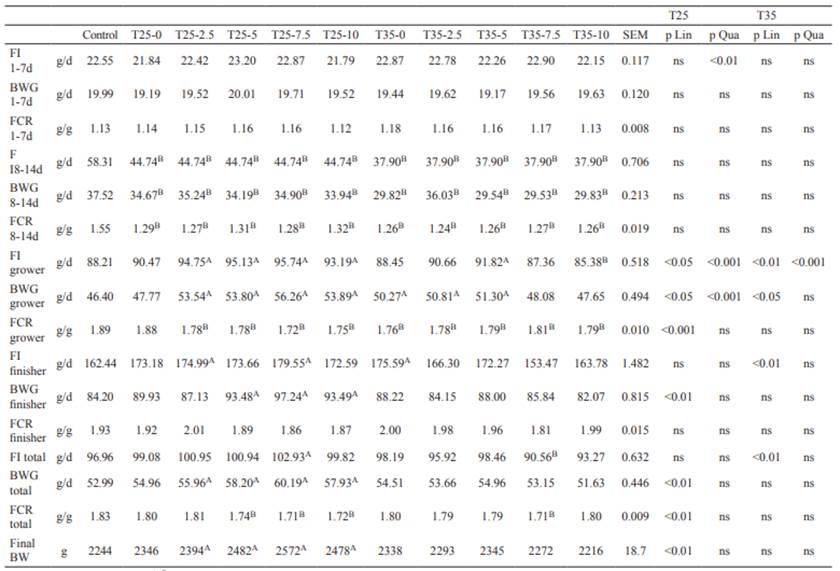
Different superscripts letters (A, B) within rows differ significantly at (p<0.05) from the control by the Dunnett’s multiple comparison test. ns: not significant.
Table 4 Immune-related organ weight and immune response after vaccination or injection of sheep red blood cells (SRBC) in broilers raised under a normal feeding program (control), or two levels of quantitative feed restriction (25 and 35%) between 8 and 14 days of age followed by refeeding high energy and protein-dense diets (0, 2.5, 5, 7.5 and 10% over the control diet) from 15 to 42 days of age.
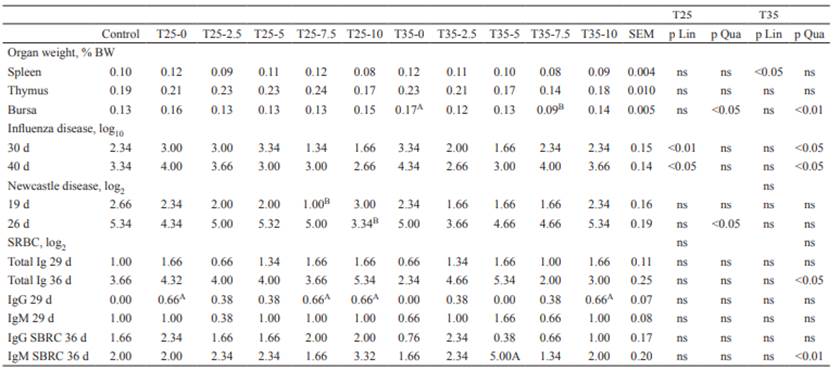
Different superscripts letters (A, B) within rows differ significantly at (p<0.05) from the control by the Dunnett’s multiple comparison test. ns: not significant.
In this regard, energy level during refeeding seems to be more important than protein content, providing that enough protein is supplied (Leeson and Zubair, 1997; Santoso et al., 1995).
Several researchers have reported the effects of feed restriction on immune-related organs and immune response of broilers (Jahanpour et al., 2015; Mahmood et al., 2007; Onbasilar et al., 2009), but information on the effects of feeding broilers nutrient dense diets following a feed restriction period on immune-related organ weight and immune response is scarce (Rahimi et al., 2015). According to the early results of Klassing (1988), it might be expected that nutritional status of the chicks could affect the immune function. However, in the present study, no remarkable effects of neither a period of feed restriction nor feeding nutrient dense diets in the re-alimentation period were observed (Table 4), which agrees with the results of Jahanpour et al. (2015), Onbasilar et al. (2009) and Rahimi et al. (2015).
Summarizing, growth performance during the overall feeding period suggests that feeding a nutrient dense diet after a period of mild feed restriction (T25) gives the best results, while increasing nutrient density of the diet after a more severe feed restriction (T35) does not improve productive results compared to a standard diet. Carcass traits and immune function are not affected by the restriction level or nutrient density during the re-alimentation period under the conditions assayed.