Introduction
Osteoarthritis (OA) has a global impact with significant economic losses in the animal population, especially among dogs and horses, as well as humans (Fox, 2010; McIIwraith et al., 2012). It is recognized as a worldwide public health problem generating disability and contributing to mortality in elderly humans. (March et al., 2016). The disease is characterized by degeneration of not only articular cartilage with different degrees of matrix fibrillation, fissure, ulceration and loss of thickness, but also other articular tissues, such as synovial membrane and subchondral bone, thus participating in the progression of OA (McIlwraith et al., 2012).
Treatment of OA poses a challenge because it needs to reduce joint inflammation and prevent damage, or to repair damaged cartilage tissue, in order to recover the characteristics of the original cartilage (Goodrich and Nixon, 2006; Goldring, 2012). Since there is currently no curative therapy, the treatment goals are pain relief and improvement of joint function. Pharmacological treatment options include nonsteroidal anti- inflammatory drugs, analgesics and intra- articular administration of corticosteroids and HA (Goldberg and Goldberg, 2010; McIlwraith et al., 2012).
Intra-articular corticosteroids are considered potent anti-inflammatory agents that suppress the immune response. However, their use remains controversial, based on their possible deleterious effects on cartilage (McIlwraith, 2010; Pekarek et al., 2011; McIlwraith et al., 2012). According to a study, intraarticular injection (IA) of steroids supplied every 12 weeks for two years in human patients with OA showed that cartilage loss and pain improvement did not differ from a placebo (Hart, 2017). Thus, its use is limited to improve the acute clinical setting (Majeed et al., 2018).
Triamcinolone acetate, triamcinolone hexacetonide, and methylprednisolone acetate are long-acting corticosteroid preparations frequently used intra-articular in human patients (Silvinato and Bernardo, 2017). The efficacy of one corticosteroid over the other remains controversial (Golding et al., 2017). However, a systematic review suggests that triamcinolone induces greater pain reduction in knee osteoarthritis compared to methylprednisolone or betamethasone (Hepper et al., 2009).
In turn, the use of IA HA for management of OA has increased. The exact mechanism of action at the cellular level is not fully understood (Goldberg and Goldberg, 2010; Legré-Boyer, 2015), but pain reduction has been related to effects on peripheral pain receptors and viscoelastic properties of synovial fluid (Goldberg and Goldberg, 2010). The anti- inflammatory effects have been associated with reduced chemotaxis and phagocytosis of neutrophils activated by HA-CD44 interactions, as well as increased synthesis of high-molecular- weight HA by synoviocytes (Goodrich and Nixon, 2006). Currently, due to lubricating, anti-inflammatory and chondroprotective properties, new products are being developed based on HA and / or together with other synergistic drugs (Bowman et al., 2018). In addition, a combined use of corticosteroids and HA has been proposed to obtain the immediate anti-inflammatory action of corticosteroid and long-term chondroprotective effects of HA (McIlwraith et al., 2012; Legré-Boyer, 2015).
Thus, the present study aimed to compare the effects of HA and TA, alone or combined, on the in vitro chondrogenic differentiation process of MSCs. The hypothesis is that HA and TA promote better in vitro chondrogenic differentiation of equine MSCs compared with the Control.
Materials and Methods
Ethical considerations
All procedures were approved by the Ethics Committee of the School of Veterinary Medicine and Animal Science from São Paulo State University “Júlio de Mesquita Filho”, UNESP, Botucatu (nº. 0049/2017 - CEUA). The products were obtained from the Life Technologies Corporation, Carlsbad, CA, USA, unless otherwise stated.
Bank cells, thawing and culture of MSCs
Equine MSCs from bone marrow of five animals aged less than 10 years previously characterized by flow cytometry from a cell bank were seeded in culture flasks (175 cm2). Each flask contained 1×106 cells and 15 mL of maintenance medium (MM) with 90% Dulbecco's Modified Eagle Medium (DMEM/F12), 10% fetal bovine serum (FBS), 1.5% penicillin-streptomycin (PenStrep), 1.2% amphotericin B (fungizone) and 0.1% Amikacine. Before the experiment, a test was performed to determine the ideal concentration and growth curve to define the differentiation day using two different concentrations.
Chondrogenic differentiation in microtube
Samples of MSCs at the third passage were placed into 12 microtubes at the final concentration of 5×105 cells/microtube. The MSCs were cultured in MM for 48 hours, then transferred to a chondrogenic differentiation medium (DM) that was prepared according to the manufacturer's recommendations. Additionally, the DM was supplemented with 1.2% Fungizone and 1.5% PenStrep.
Once the differentiation was underway, the microtubes were equally divided into 4 treatment groups (each group in technical triplicate): Control, HA (10%) (Termofrio Laboratory, São Carlos, SP, Brazil), TA, and HA/TA (Table 1). The concentrations of the drugs were based on previous studies (Siengdee et al., 2015; Wernecke et al., 2015). Each microtube had 5×105 cells per 500 μL of differentiation medium and its respective treatment. The samples were centrifuged at 600 g for 5 minutes and incubated at 37 °C and 5% CO2 for 14 days. The DM with each treatment was replaced every 72 hours.
Histological and immunohistochemical (IHC) evaluations
After culturing, the pellets were processed for histological analysis. Sections were stained with hematoxylin-eosin (H&E), Alcian blue (AB) (pH 2.5) and toluidine blue (TB), and IHC for type II collagen was conducted.
The IHC samples were evaluated for the presence of type II collagen in the cytoplasm and ECM. Histological sections of 4 mm were obtained from the pellets in positive charged slides (Amitel®), which were placed 15 minutes on the stove at 60 °C, deparaffinized in xylene, and rehydrated in decreased ethanol concentrations. After washing under running water for 5 minutes, the sections were exposed to heat in a pressure cooker with citrate buffer solution (pH 7.0) at 125 °C for 25 minutes in order to induce them to epitope retrieval. Blocking of endogenous peroxidase was performed with PBS + 3% hydrogen peroxide in dark luminescence for 30 minutes. Then, the sections were washed in a Tris solution with Ph 7.4. Nonspecific ligation sites were blocked with a 3% solution of nonfat dry milk for 60 minutes. Sections were then incubated (overnight) by using primary antibody (1:300 type II collagen, Anti-Collagen II ab34712, Abcam, Cambridge, MA, USA). Second incubation was performed with the secondary antibody (1:300, polyclonal goat anti-rabbit IgG-HRP p0448, Dako) for 90 minutes. The slides were revealed with chromogen 3,3´-diaminobenzidine (DAB), and counter- stained by using Harris hematoxylin.
The IHC analysis was carried out independently by two researchers. The samples were evaluated semi-quantitatively as follows: 1: ≤ 25% of labeled cells, 2: 26-50%, 3: 51-75% and 4: ≥ 75%; which were considered respectively: 1) unstained, 2) slightly stained, 3) moderately stained, and 4) intensely stained (Amaral, 2005). The total number of cells from five fields were randomly counted. The images were captured by an optical microscope (Zeiss®, Germany) with 40x objective lens. The captured images were analyzed using the AxioVision 4.8 software.
Statistical analysis
The Kolmogorov-Smirnov method was employed to assess normality. The reliability of IHC measurements taken by the researchers was analyzed by an unpaired Student’s t test. One- way ANOVA was used for comparison among four groups. Tukey was used as post-hoc test. Differences were considered statistically significant when p<0.05.
Results
Cell and pellet evaluation
Cell viability was ≥ 80%. Adhesion to the flask was obtained at 48 hours. Fibroblastoid morphology was visualized after five days of culturing and reached 80% confluence in 11 days. Macroscopically, the concentration of 1×105 cells generated a 2 mm diffuse pellet, while that of 5×105 cells generated a 4 mm consistent pellet (Figure 1).
Macroscopic evaluation
In the HA and HA/TA groups the pellet underwent conformation loss, and spread at the bottom of the microtubes. The control and TA group maintained the compact and homogeneous pellet shape. In all groups, there was no visible pellet growth.
Histological and IHC evaluations
The HA/TA Group showed a cellularity increase compared to the others (Figure 4). The HA/TA Group and more specifically the HA Group showed rounded cells located within the lacunae. Nevertheless, the control and TA groups did not exhibit chondrocyte-like morphology. The round nuclear shape predominated in the cells of all groups. The ECM presented a homogeneous and compact aspect, which appeared more intense in the Control and TA groups (Figure 2).
The TA Group presented moderate production of Glycosaminoglycan compared to the Control Group. Glycosaminoglycan production was mild in the HA/TA and not detectable in the HA Group. In turn, the Control Group showed intense staining (Figure 3).
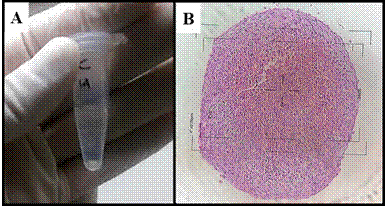
Figure 2 Cellular histomorphology. (A) Control showing the presence of ECM (black arrow) surrounding fibroblast-like cells and a few rounded cells. (B) TA Group displaying increased cellularity and presence of some rounded cells with eosinophilic cytoplasm and abundant ECM (black arrow). (C) HA Group showing ECM and discrete number of chondrocyte-like cells (white arrow) within lacunae (black arrow). (D) HA/TA Group presenting small amount of ECM and chondrocyte-like cells (black arrow) located within lacunae. H&E staining.
There were no statistically significant differences for IHC measurements taken by the researchers (p≥0.7331). As to the percentage of cells stained by IHC, intense staining was poorly represented. The Control Group was moderately stained, TA and HA groups were slightly stained while the HA/ TA group was unstained (Figures 4 and 6). For all groups, the highest percentage of stained cells had a slight staining intensity. Furthermore, the TAGroup had the highest percentage of intensely stained cells when compared to the other groups (Figure 5).
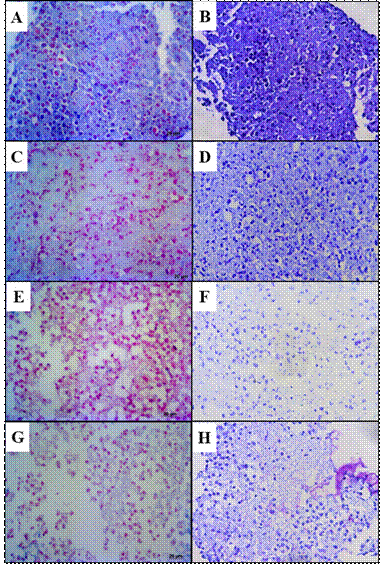
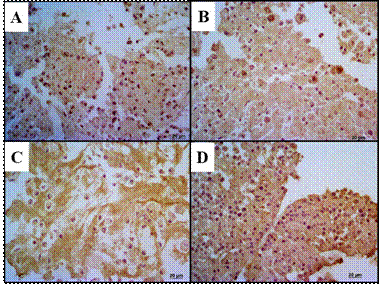
Figure 3 Glycosaminoglycan detection by AB (left column) and TB (right column) (A-B) Control showing intense staining. (C-D) TA Group with moderate staining. (E-F) HA Group with no staining. (G-H) HA/TA Group with slight staining.
However, there were no statistically significant differences among groups (p=0.8190).
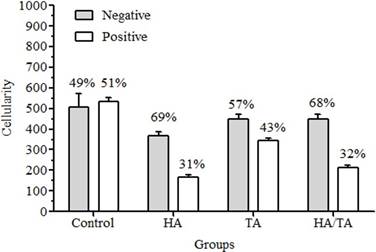
Figure 4 IHC evaluation for type II collagen (cellularity and immunoreactivity) according to cell-treatment group. Values are expressed as mean ± standard deviation.
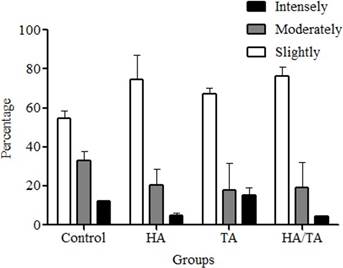
Figure 5 Immunoreactivity percentage of type II collagen-positive cells in cell-treatment groups according to staining intensities. Values are expressed as mean ± standard deviation.
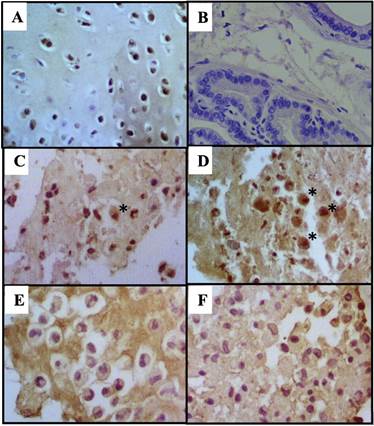
Figure 6 IHC for type II collagen for cells (A) Positive control: Articular condrocytes (B) Negative control: Prostate (C) Control with moderate staining. (D) TA group with slight staining (*field with few intensely stained cells). (E) HA group with slight staining and, (F) HA/TA group considered unstained. 100x.
The ECM of the HA Group and more specifically the HA/TA Group were more intensely stained than TA and Control groups. (Figure 7). Non-specific staining results were discarded.
Table 2 Immunohistochemistry score for type II collagen according to extracellular-matrix treatment groups.

*Score: 1= unstained; 2 = slightly stained; 3 = moderately stained; 4= intensely stained.
Discussion
Each of the several in vivo models, in vitro experimental systems and testing of intra- articular medication present its own advantages and disadvantages (Jo et al., 2014; Mak et al., 2016; Grodzinsky et al., 2017). In the present study we chose the cell-culture testing because of the possibility of microenvironmental control, characterization, homogeneity of the samples, and in vitro modeling of in vivo conditions (Freshney, 2010).
In order to obtain material with a phenotype similar to native articular cartilage, the pellet culture system was chosen. This system allows cell-to-cell interaction, similar to the pre- cartilage condensation of stem cells during embryonic development (Yang et al., 2004; Bhumiratana et al., 2014), and increases the efficiency of cell seeding and formation of ECM rich in type II collagen (Larson et al., 2002). However, some authors have reported that MSCs cultured and differentiated in a pellet system do not always present a chondrocyte phenotype, but rather express some specific markers of hypertrophic tissue. This affirmation was corroborated in the present study, in which only the HA Group showed the positive phenotype. Despite disagreement among some researchers, this system has been recommended for clinical use and for elucidating details about chondrogenesis in the early stages (Yang et al., 2004).
MSCs subjected to chondrogenic differentiation in a pellet culture system, in general, have a decreased proliferation rate (Baksh et al., 2004). Thus, to guarantee a sufficient number of MSCs for the evaluations, the present study used a high cell concentration, which was determined based on the pellet culture test. Similar concentrations were employed in studies that differentiated MSCs isolated from different sources into chondrocytes, adipocytes and osteocytes (Lovati et al., 2011; Schuurman et al., 2016).
Macroscopically, the loss of pellet conformation that occurred in the HA and HA/ TA groups are probably related to viscosity and dense consistency of HA. A crosslinked HA with molecular weight of 6,000 kDa was used in the present study. There is heterogeneity in the estimates of HA efficacy among the studies, but high-molecular-weight HA seems more effective (Lo et al., 2003; Wang et al., 2004). Histologically, only cells in HA and HA/TA groups exhibited chondrocyte-like morphology, including round-shaped cells located in lacunae (McGeady et al., 2017). Probably, the hydrophobic capacity of HA that provides a stiffness and viscous consistency of the medium favored a better cellular differentiation. Other studies have found that the use of scaffolds composed of HA may favor chondrogenic differentiation of MSCs (Awad et al., 2004; Matsiko et al., 2015). The cells of control and TA groups did not exhibit chondrocyte-like morphology. A homogeneous ECM and some round cells inside lacunae were observed in the HA/TA group. The HA group presented scant ECM surrounding a large number of round cells inside lacunae. A decrease in chondrogenic differentiation of MSCs cultured in hydrogel without HA or HA mixed with other compounds was also observed in a study using umbilical-cord-blood-derived MSC composites and four types of hydrogels (Chung et al., 2014).
Glycosaminoglycan production assessed by AB staining was decreased in all the groups compared to the Control. The moderate response in the TA Group probably was influenced by the time of exposure to the corticosteroid. An in vitro study on chondrocytes from dogs exposed to TA at different time-points concluded that continuous exposure to TA slightly reduced Glycosaminoglycan (14 days), which was attributed to corticosteroid receptor downregulation (Jansen et al., 2016). On the other hand, glucocorticoids are important for the maintenance of cartilage homeostasis, and are also used in the process of chondrogenic differentiation of MSCs in vitro (Florine et al., 2013; Randau et al., 2013; Jakobsen et al., 2014).
The response to Glycosaminoglycan was slight in the HA/TA Group, but negative in the HA Group. However, MSCs submitted to chondrogenic differentiation in an enriched hydroxyapatite scaffold environment resulted in increased staining of the Glycosaminoglycan compared to the control at day 9 of differentiation (Wu et al., 2010). Another study also observed Glycosaminoglycan accumulation in the HA hydrogels with MSCs (Zhu et al., 2014). Among the factors that may have influenced Glycosaminoglycan production are HA presentation, which was liquid and not by scaffold, and the fact that the cells were not initially centrifuged. A study on in vitro cartilage formation by human MSCs mentioned that chondrogenesis can be induced by artificially condensing the cells by centrifugation (Sekiya et al., 2002).
The type II collagen expression in the cytoplasmic level showed moderate increase in the Control Group, slight elevation in the TA and HA groups, and no increase in the HA/TA Group. This suggests an adverse effect on type II collagen caused by the medications, which may be related to medication concentration or even continuous exposure. Another hypothesis is that the expression of hypertrophic markers like type X collagen is higher in MSCs differentiated in a pellet culturing system (Yang et al., 2004). A study on the effects of various glucocorticoids (dexamethasone, prednisolone, triamcinolone) on cultured equine chondrocytes found that glucocorticoids affect numerous genes involved in the cartilage matrix and low doses cause minimum negative effects on the ECM degenerative process (Richardson and Dodge, 2003). However, the use of intraarticular TA in horses decreased expression of type II collagen in synovial fluid (Céleste et al., 2005).
On the other hand, ECM was positively stained by type II collagen in all groups, in which the HA/TA Group was intensely stained, the HA Group moderately and the other groups slightly. A study showed that incorporation of HA methacrylate into human chondrocytes encapsulated in gelatin-methacrylamide hydrogels produced more rounded cell morphologies, enhanced chondrogenesis, increased quantity and distribution of the newly synthesized ECM, and high-intensity staining by type II collagen. It has been suggested that hyaluronic acid methacrylate can be a potent modulator of differentiation (Levett et al., 2014).
The current study presented some limitations, most significantly the non-evaluation of the genetic panel by PCR testing. Additionally, the low number of samples may have influenced the statistical analysis. Although the main goal of the study was to test the medications in their original formulations for intra-articular administration, a different approach would be to incorporate them in a scaffold.
In conclusion, histological analysis reveals that HA stimulates chondrocyte-like morphology in the different groups, but with low expression of specific cartilage molecules; TA increases the formation of a more homogeneous and organized extracellular matrix.