INTRODUCTION
The jagua (Genipa americana L.) is a neotropical plant belonging to the Rubiaceae family. It is native to the Amazon basin and grows naturally from tropical to subtropical forests in Latin America (Brauch et al., 2016; Francis, 2000; Náthia-Neves & Meireles, 2018). The jagua has a straight stem, dark green leaves, golden yellow flowers, and a fruit in the shape of a green ovoid berry, which turns greyish when it ripens and has a light brown pulp that envelops the center of the seed (Moura et al., 2016). In Colombia, this species is distributed in the departments of Antioquia, Quindío, Risaralda, Amazonas, Cundinamarca, and Boyacá, where it is called jagua or huito (Pinto-Ruiz et al., 2018). Said species is used both for its wood characteristics and its fruit -which has various medicinal, nutritional, and cosmetic attributes- thus leading to an extractive activity without any care for conservation or knowledge about the species (Santos et al., 2011; Silva et al., 2018). Jagua trees are fast-growing and a good option of economic importance for small farmers. Nevertheless, even with all the knowledge about the productive potential of G. americana in tropical regions, the available information is scarce, especially regarding exploration, production, and initial growth studies (Paiva et al., 2019; Santos et al., 2011). Therefore, the develop- ment of evaluation studies of genetical diversity can yield valuable information for both plant improvement and commercial production programs (Jesus et al., 2019). The wide geographic distribution of G. americana, mainly in different forests in wet tropical and subtropical regions from Brazil to Mexico, implies a plant with high plasticity and adaptability (Gonçalves et al., 2013; Santiago & Paoli, 2007). In addition, the different responses reported for this plant through various environmental conditions suggest a variation of G. americana biotypes as it adapts to the specific requirements of the site where it grows (Gonçalves et al., 2013; Petit & Montagnini, 2004; Santiago & Paoli, 2007). Consequently, the evaluation of biotypes of the same species collected in contrasting environments would be expected to obtain a differential response of genotypes under particular environmental evaluation conditions. In the case of G. americana, studies evaluating the physiological efficiency both indirectly (Barbosa et al., 2007; Mielke et al., 2003) and directly (Petit & Montagnini, 2004; Santiago & Paoli, 2007; Santiago et al., 2018; Silva et al., 2018) have been reported. However, this type of work is still scarce for this species. Growth analysis has been based on the descriptive examination of variables or functional polynomial expressions (Paine et al., 2012). Thus, understanding the functioning of agricultural systems, as complex as they are, requires constructing models of the system’s efficiency based on environmental parameters, soil components, and interactions between components (Arredondo & Castañeda-Sánchez, 2020). Therefore, this study aims to evaluate the growth of the five origins of G. americana in the vegetative stage by adjusting non-linear models to growth variables.
MATERIALS AND METHODS
Location
This study was conducted in the village of El Encanto (6° 78´ 26.6¨ N; 75° 09´ 09.3 W, and an elevation of 1000 masl), in the municipality of Amalfi, department of Antioquia, Colombia, which is located in a tropical wet forest life zone (T-wf), with an average annual rainfall of 1968 mm and a bimodal distribution, in which there is a rainy season (March-April) and a drought season (August-September), with an average yearly temperature of 24.2 °C and a relative humidity of 74.6%.
Characteristics of the study site
The properties of the soil where the study was conducted were as follows. The textural class was sandy clay, and the composition was: pH: 5.1; MO 3.1%; P: 2 mg kg-1.soil; S: 5 mg.kg-1.soil; Ca: 0.75 cmolc.kg-1; Mg: 0.30 cmolc.kg-1; K: 0.08 cmolc.kg-1; CICE: 1.8 cmolc.kg-1; Fe: 101 mg.kg-1; Mn: 3 mg.kg-1; and Cu: 1 mg.kg-1; Zn: 1 mg.kg-1. The type of landscape in this area is mountainous, with ridges, rows, beams, hills, and intramontane valleys, with lithological materials such as quartz diorite, an intrusive igneous rock corresponding to the Antioquia batholith (the study was located under this material with hill-type slopes). Lower hills with little slopes (12-25%) are towards the center of the Porce river; in the more distal parts of the nearby valley, there are hills with steeper slopes (25-50%) and underdeveloped soils (Jaramillo, 1989). Table 1 summarizes the annual mean environmental variables registered for the evaluation period between 2014 and 2019.
Table 1 Climatic characteristics of the municipality of Amalfi (Antioquia), place of evaluation of the Genipa americana origins. The data were taken from the EPM El Mango Porce II weather station (2014-2019).
| Parameter | Year | |||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Mean temperature (ºC) | 23.3 | 23.6 | 23.5 | 22.7 | 22.5 | 22.9 |
| Maximum temperature (ºC) | 25.0 | 25.6 | 25.3 | 24.8 | 24.4 | 24.8 |
| Minimum temperature (ºC) | 21.3 | 21.4 | 21.5 | 20.8 | 20.6 | 21.0 |
| Relative humidity (%) | 79.5 | 79.6 | 81.4 | 81.8 | 84.0 | 84.2 |
| Annual rainfall (mm) | 1776.3 | 1098.3 | 2168.7 | 1415.0 | 1843.7 | 1790.1 |
Experimental conditions. The treatments consisted of five (5) origins of G. americana L., called Vigía del Fuerte, Porce (Amalfi), Rafael, San Luis, and Chigorodó (Figure 1). Table 2 lists the main environmental characteristics of the five regions or origins of the evaluated genotypes.
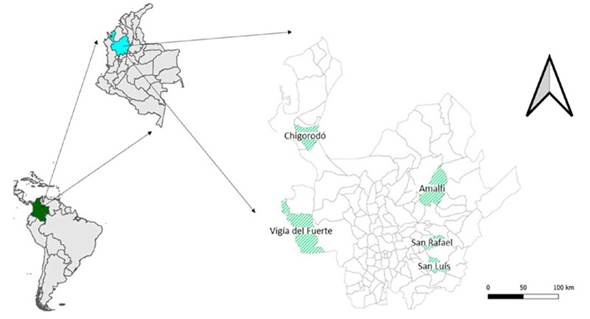
Figure 1 Geographical location of the municipalities from which the five accessions of Genipa americana come. Place of evaluation: Amalfi, Antioquia, Colombia.
Table 2 Edaphoclimatic characteristics of the areas of origin of the Genipa americana accessions
| Place of origin | Temperature (°C) | Rainfall (mm/year) | RH(1) % | Edaphic characteristics |
|---|---|---|---|---|
| San Luis | 23.76 | 4579 | 67 | Colluvial relief, strongly undulating. The slopes vary between 25-70% of inclination. Soils developed from colluvial deposits, with good drainage and moderately coarse textures. |
| San Rafael | 21.94 | 3965 | 68 | The colluvial relief is strongly undulating, with slopes greater than 20%. The soils derived from igneous rocks are deep, well-drained, and with moderately fine textures. |
| Chigorodó | 28.36 | 3800 | 65 | Alluvial fan geoforms. The relief varies from flat to slightly flat, with slopes of less than 3%. These are soils developed from mixed alluvium, with a good level of depth and moderately fine textures. |
| Vigía del Fuerte | 28.42 | 5100 | 74 | Flat relief with slopes of less than 3%. Soils developed from fine alluvium and accumulations of organic matter, with fluctuating ground-water tables and medium and fine textures. |
| Porce | 22.6 | 3641 | 64 | Strongly undulating. Steep reliefs with slopes between 25-75%. Soils developed from undifferentiated metamorphic rocks, with volcanic ash deposits, good drainage, and fine textures. |
(1) Relative humidity (RH)
Source: IGAC (2007)
The germination of seeds was carried out in the sand, with daily irrigation for 45 days until the seedlings were between 2 and 3 cm high. The seedlings were transplanted into bags with a substrate distributed in equal proportions of sand, soil, and organic matter, and they were kept under shade conditions until they reached a height between 30 and 40 cm. Sowing was carried out in June 2015 within 7 x 7 m. Each accession of G. americana was randomly distributed in plots of 2400 m2 with 49 plants, considering the plant as the experimental unit (replication), for a total experimental area of 12 000 m2.
Evaluated variables. The total height of the plants was determined with a measuring tape if they were less than 3 m, and with a clinometer (Suunto Tandem/360PC/360R®) if they were taller. The diameter was recorded at 0.1 m from the ground (D10) using a caliper in plants with a height of less than 3 m and at 1.3 m (D130) using a measuring tape in taller plants (Diameter at Breast Height (DBH). Eight evaluations were carried out within a period of six months, namely in July and October 2015, March and August 2016, June 2017, March 2018, April 2019, and May 2020.
Statistical analysis
The growth of the trees from each origin was modeled through the behavior of the plants’ height, D10, and D130 over time. Additionally, each variable was taken as a response according to the origin factor, with its five levels, in order to evaluate significant differences (p<0.05) in their means. Growth comparisons between the different accessions were performed independently at each measurement. The growth over time was evaluated by means of nonlinear models. After the Shapiro-Wilk normality test, it was found that the residuals distribution of the analysis of variance models did not exhibit normality for plant height and diameters D10 and D130 (p = 2.2e-16). Therefore, in order to compare the variables between the different sources, the non-parametric Kruskal Kruskal-Wallis test was used; the post-hoc test used Fisher's least significant difference criterion, and the Bonferroni adjustment method was used in the agricolae package on the R software (de Mendiburu, 2021).
Functional growth analysis
The fit and predictive capacity of four nonlinear functional models, which are frequently used to represent plant growth, were evaluated. The mathematical expressions for these models are described below:
where yR, yL, yW, and yG refer to the response variables (height and diameters at 0.10 and 1.30 m) estimated using Richards (1), logistic (2), Weibull (3), and Gompertz (4) non-linear models, respectively. α is the value of the maximum growth (asymptote), b is a positive value that depends on the initial condition of the population, κ is growth rate, x is time, and c is the intercept in the y-axis (Karadavut et al., 2010). The c parameter was considered, with (Gompertz-4 and logistic-4) and without it (Gompertz-3 and logistic-3), only in the logistic and Gompertz models.
The Gompertz and logistic models were evaluated with four and three parameters; the Richards and Weibull models were adjusted with three parameters since the c parameter was not significant within them. The estimation of the parameters of the models with the best fit to the data was determined through the Akaike (AIC) and Bayesian (BIC) information criteria (Motulsky & Christopoulos, 2003).
The prediction capacity of each model under study was evaluated using the RMSPD criterion (root mean squared predictive difference of errors). If the RMSPD value is closer to zero, the model will have a better prediction (Hastie et al., 2009). All of the above was performed using R software (de Mendiburu, 2021).
RESULTS
Descriptive growth analysis
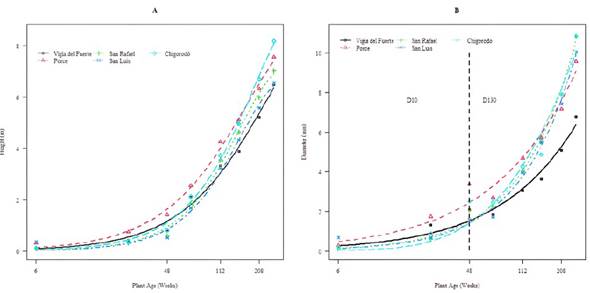
All the accessions show a stable trend in weeks 6 to 48 for plant height, whereas, for the stem diameter, these show an increasing behavior. Plants aged between 48 and 70 (all the accessions) exhibited an abrupt increase in growth rates, reaching a maximum peak at the age of 70 weeks in the plant height variable and possibly also in D10, since, at that age, the diameter was measured at 1.30 m from the ground (Figure 2). However, the growth rates in the plants of the Porce, Chigorodó, and Vigía del Fuerte accessions were higher compared to those of the others, which would explain the greater height and D10 reached by them. Plants aged between 70 and 150 weeks of all the accessions showed a decrease in the weekly growth rates, but they stabilized after 150 weeks of age. However, in the plants of the Chigorodó accession, the growth rate continued to be higher in comparison with the others, which explains that, when these plants reached an age of 262 weeks, they showed the highest significant height (8.18 m); they were followed by the Porce accession (7.56 m), San Carlos (7.02 m), San Luis (6.55 m), and Vigía del Fuerte (6.49) (Figure 2, Table 3).
At six weeks of development, the different accessions already showed significant differences (p<0.05) regarding plant height and stem diameter. With six weeks of development, the Porce accession stood out with the highest plant height and the largest trunk diameter, surpassing the other four origins (Vigía del Fuerte, San Rafael, San Luis, and Chigorodó). This behavior remained constant for these variables in the following evaluations: weeks 26, 48, 70, 112, and 208 (Table 3). On the contrary, in the last evaluation, the Vigía del Fuerte origin was the genetic material with the lowest average height (6.49 m) (Table 3). For the stem diameter variable (D10), recorded for up to 48 weeks (1 year) of development, the material from Porce stood out as the one with the largest diameter (3.37 cm), significantly surpassing the rest of the origins. From week 70 until the evaluation carried out at week 212 (4 years), it was observed that Porce and Chigorodó stood out as the origins with the highest growth in stem diameter. However, in the last evaluation, conducted five years after establishment, Chigorodó, San Rafael, and San Luis showed the highest values for this variable, significantly surpassing Porce and Vigía del Fuerte (Table 3).
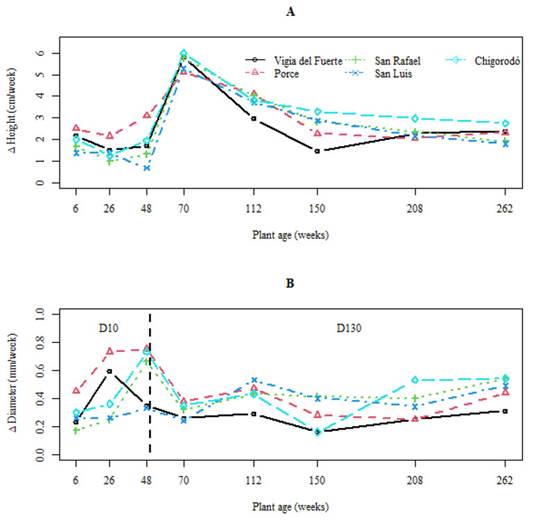
Figure 2 Weekly growth in plant height (A) and stem diameter (B) for the five accessions of Genipa americana. D10: Stem diameter at 0.10 m from the ground until week 48. D130: Stem diameter at 1.30 m from the ground between 48 and 262 weeks.
Table 3 Plant height and stem diameter 0.10 and 1.30 m from the ground for the five provenances of Genipa americana during the vegetative phase
| Origin | Plant age (week) / Evaluation date | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 | 26 | 48 | 70 | 112 | 150 | 208 | 262 | |
| 06/11/2015 (24)(1) | 10/30/2015 (44) | 03/31/2016 (13) | 09/01/2016 (35) | 05/20/2017 (25) | 03/17/2018 (11) | 04/27/2019 (17) | 05/05/2020 (19) | |
| Plant height (m) | ||||||||
| Vigía del Fuerte | 0.13± (0.02) v(2) | 0.43± (0.14) s | 0.81± (0.27) q | 2.09± (0.28) n | 3.33± (0.58) l | 3.88± (0.99) j | 5.21± (1.18) g | 6.49± (2.75) e |
| Porce | 0.31± (0.11) tu | 0.74± (0.13) q | 1.42± (0.26) p | 2.54± (0.39) m | 4.26± (0.66) i | 5.12± (0.81) g | 6.31± (1.28) de | 7.56± (1.38) b |
| San Rafael | 0.10± (0.01) w | 0.30± (0.10) u | 0.59± (0.22) r | 1.86± (0.29) no | 3.55± (0.54) kl | 4.62± (0.55) h | 5.99± (0.85) e | 7.02± (1.58) c |
| San Luis | __(3) | 0.36± (0.08) tu | 0.51± (0.12) r | 1.67± (0.18) op | 3.23± (0.52) l | 4.32± (0.54) i | 5.58± (0.81) f | 6.55± (1.31) cd |
| Chigorodó | 0.12± (0.02) vw | 0.37± (0.13) st | 0.80± (0.25) q | 2.12± (0.38) n | 3.72± (0.58) jk | 4.97± (0.83) g | 6.7± (1.00) c | 8.18± (1.09) a |
| Stem diameter (cm) | ||||||||
| D10(4) | D130 (5) | |||||||
| Vigía del Fuerte | 0.14± (0.05) l | 1.31± (0.32) f | 2.08± (0.70) c | 1.84± (0.44) op | 3.04± (0.72) l | 3.63± (1.06) k | 5.07± (1.32) g | 6.77± (1.65) e |
| Porce | 0.27± (0.07) j | 1.73± (0.27) d | 3.37± (0.54) a | 2.69± (0.43) m | 4.67± (0.90) h | 5.72± (1.32) f | 7.16± (1.75) e | 9.56± (2.20) b |
| San Rafael | 0.10± (0.02) m | 0.59± (0.26) i | 2.04± (0.73) c | 2.23± (0.49) no | 4.04± (0.81) ij | 5.59± (0.99) f | 7.92± (1.33) cd | 10.83± (1.55) a |
| San Luis | ___ | 0.68± (0.14) h | 1.57± (0.27) e | 1.71± (0.37) p | 3.95± (0.52) j | 5.47± (0.74) g | 7.42± (1.12) d | 10.04± (1.35) a |
| Chigorodó | 0.18± (0.06) k | 0.89± (0.35) g | 2.5± (0.71) b | 2.46± (0.53) mn | 4.26± (0.74) i | 4.86± (1.56) gh | 7.91± (1.27) c | 10.85± (1.41) a |
Functional models of growth
Table 4 shows the magnitude taken for the adjustment criteria (BIC and AIC) and prediction (RMSPD) for the different models (Gompertz-3, logistic-4, logistic-3, and Weibull) adjusted for the height growth of the plants according to their origin. Considering the BIC and AIC information criteria, the Weibull model was the one with the lowest value for these predictors in each of the origins, so it was considered the one that best describes plant growth for all origins. Likewise, the best fit reported by the Weibull model indicates that it has a high predictive capacity, given its RMSPD<1.18 for the plant height variable of G. americana, in comparison with the other three evaluated models. The theoretical Weibull model parameters that allow estimating the growth of plants according to their origin are presented in Table 5. According to the origin, the differences found confirm the dissimilarity in plant development for the vegetative phase, thus requiring specific models.
Table 4 Statistical criteria for evaluating the fit and predictive capacity of different models at the height of Genipa americana plants from different accessions
| Origin | Criteria | Growth model | |||||
|---|---|---|---|---|---|---|---|
| Gompertz-4 | Gompertz-3 | Logistic-4 | Logistic-3 | Weibull | Richards | ||
| Vigía del Fuerte | BIC (1) | -(4) | 1193.80 | 1193.80 | 1210.00 | 1183.60 | 1199.80 |
| AIC (2) | - | 1178.20 | 1174.30 | 1194.40 | 1168.00 | 1180.20 | |
| RMSPD (3) | - | 1.19 | 1.18 | 1.22 | 1.18 | 1.53 | |
| Porce | BIC | 1109.90 | 1113.40 | 1112.20 | 1156.60 | 1097.40 | 1119.50 |
| AIC | 1089.40 | 1096.90 | 1091.60 | 1140.20 | 1081.00 | 1098.90 | |
| RMSPD | 0.81 | 0.82 | 0.81 | 0.86 | 0.80 | 0.90 | |
| Chigorodó | BIC | 824.90 | 831.70 | 827.50 | 888.50 | 803.10 | 835.40 |
| AIC | 805.20 | 815.90 | 807.80 | 872.80 | 787.40 | 815.70 | |
| RMSPD | 0.69 | 0.70 | 0.65 | 0.76 | 0.68 | 0.70 | |
| San Rafael | BIC | 858.10 | 855.20 | 867.20 | 897.60 | 837.70 | 856.60 |
| AIC | 838.50 | 839.50 | 847.80 | 881.90 | 822.00 | 837.00 | |
| RMSPD | 0.74 | 0.74 | 0.75 | 0.78 | 0.72 | 0.91 | |
| San Luis | BIC | 699.30 | 699.40 | 702.00 | 735.60 | 684.30 | 694.20 |
| AIC | 680.40 | 684.30 | 683.10 | 720.50 | 669.20 | 675.40 | |
| RMSPD | 0.67 | 0.68 | 0.68 | 0.72 | 0.67 | 0.91 | |
(1) Bayesian information criteria. (2) Akaike's information criteria. (3) Mean square root of the prediction errors expressed in cm. (4) No convergence.
Table 5 Significant parameters (p <0.01) of the Weibull model adjusted to the plant height and stem diameter vegetative growth variables of five origins of Genipa Americana
| Origin | Parameter | Weibull model (f(x) = α e -eb(log(x)-k) | ||
|---|---|---|---|---|
| α (1) | b (2) | k (3) | ||
| Plant height (H) | ||||
| Vigía del Fuerte | 9.01 | 1.29 | 223.54 | f(H) = 9.01 * e -e1.19(log(x)-223.54) |
| Porce | 9.05 | 1.27 | 171.04 | f(H) = 9.05 * e -e1.27(log(x)-171.04) |
| San Rafael | 7.43 | 1.75 | 150.31 | f(H) = 7.43 * e -e1.75(log(x)-150.31) |
| San Luis | 6.98 | 1.76 | 152.65 | f(H) = 6.98 * e -e1.76(log(x)-152.64) |
| Chigorodó | 10.03 | 1.55 | 190.10 | f(H) = 10.03 * e -e1.55(log(x)-190.1) |
| Stem diameter (D) | ||||
| Vigía del Fuerte | 52.20 | 0.87 | 2703.49 | f(H) = 52.20 * e -e0.87(log(x)-2703.49) |
| Porce | 93.18 | 0.80 | 4579.37 | f(H) = 93.18 * e -e0.80(log(x)-4579.37) |
| San Rafael | 134.23 | 1.17 | 2214.44 | f(H) = 134.23 * e -e1.17(log(x)-2214.44) |
| San Luis | 101.64 | 1.18 | 1797.85 | f(H) = 101.64 * e -e1.18(log(x)-1797.85) |
| Chigorodó | 129.72 | -0.34 | 4181.16 | f(H) = 129.72 * e -e-0.34(log(x)-4181.16) |
(1) Upper asymptote clarifies that it was not reached, since the plants were in a vegetative stage and in active growth. In this case, it is related to the maximum growth achieved. (2) Slope of the curve. (3) Inflection point indicating the change in growth rates. (x) Plant age in years.
From the parameters indicated in Table 5, the growth curves were estimated for both plant height and trunk diameter variables, as shown in Figure 3. A slow growth phase was evidenced until week 48, from which an increase in the slopes of the curves of all origins was observed, and it can be said that it corresponds to the exponential growth phase. Since the evaluations were made during the vegetative growth phase, it was not possible to observe the maximum asymptote, which has a slowdown in growth rates. It is why the typical sigmoidal curve cannot be observed. Additionally, the higher growth exhibited by the plant accessions of Chigorodó and Porce, medium in San Rafael, and low in those of Vigía del Fuerte and San Luis was confirmed.
DISCUSSION
Plant growth is a parameter used to evaluate conditions such as genotype-environment adaptation determined from height and diameter growth rates. Works carried out by Paiva et al. (2019) reported average annual increases of 10.2 cm in height and 3.10 mm in diameter for G. americana for the first year of growth. The results obtained in this study indicate that G. americana showed good development. The cultivar with the highest annual growth for the variables of plant height (42 cm. year-1) and stem diameter (7.1 mm. year-1) was Porce, while San Rafael was the one with the lowest increase (31 cm. year-1 and 7 mm. year-1).
Authors such Silva et al. (2018) and Petit & Montagnini (2004) highlighted that G. americana is a species that presents high phenotypic plasticity. This allows it to adapt and grow adequately in contrasting environments. In this sense, their growth and development behavior allows highlighting the adaptability of G. americana, starting from the flexibility in its genetic structure, which can generate changes in response to environmental conditions and thus show great variability between populations (Francis, 2000).
According to Jesus et al. (2019), although G. americana occurs in natural reserves and indigenous lands, agricultural expansion has caused a considerable loss of genetic variability in this species. It is expected that these variations are due to genotypic differences. In this regard, Silva et al. (2018) evaluated the diversity of 150 accessions from wild populations collected in only three states of Brazil, finding groups of diversity. Similar studies were conducted by Jesus et al. (2019) to evaluate the genetic diversity in the state of Sergipe (Brazil). These authors reported that the genetic similarity index (Jaccard index) between individuals was 60.4% on average, while the greatest distance of similarity obtained was observed between individuals (83.6 % ± 0.03), as well as the lowest genetic similarity between individuals (36.5 % ± 0.02). This indicates an intermediate genetic diversity, as found by Cardoso et al. (2019) in a study carried out in natural populations of G. americana in the Brazilian state of Sergipe. As previously indicated, differences were found in this study with regard to plant development for the variables of plant height and DBH during the vegetative phase according to the origin. Thus, it was necessary to adjust a growth curve for each origin (Figure 3) and thus adjust a curve for each of the evaluated populations, which indicates the variability between the origins used.
The behavior of growth curves can vary according to the organisms, the phenotype, and the environment to which species are exposed. This is the reason why it is important that the curves of the models used to evaluate whether the growth of species fit the real data trend and that its parameters allow a biological interpretation (Karadavut et al., 2008). The Weibull function is characterized by being highly flexible due to its shape and scale parameters; both parameters, according to Yang et al. (1978), have a positive numerical domain between zero and infinity. These authors have used the Weibull distribution function at the forest level to make predictions of timber yield, incorporating variables such as DBH, height, and age. They recommend its use due to its simplicity of application and straightforward interpretation. Díaz et al. (2017) conclude that the Weibull model applied to the diameter categories of Pinus cooperi is appropriate and has a good fit. Among the advantages of the Weibull distribution are its simple handling and flexibility to adopt different shapes with different degrees of bias; it can also be analytically integrated with the biological interpretation of its parameters.
Implementing models to predict growth has proven to be a valuable tool to describe plant growth accurately, continuously, and depending on the phenological stages. Hence, the Weibull model best describes and adjusts the vegetative stage of G. americana, thus allowing to complement the modeling of the life cycle of this species in future research.