Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista ION
Print version ISSN 0120-100X
Rev. ion vol.26 no.2 Bucaramanga July/Dec. 2013
removal of Cr3+ and Ni2+ from electroplating wastewater
Víctor Darío Contreras Niño1; Yenny Paola Castillón Bello2; Carlos Alberto Ríos Reyes1*;
Luz Yolanda Vargas Fiallo2
1 Escuela de Geología. Universidad Industrial de Santander (UIS), Cra. 27 Calle 9, Bucaramanga, Colombia
*carios@uis.edu.co
2 Escuela de Química. Universidad Industrial de Santander (UIS), Cra. 27 Calle 9, Bucaramanga, Colombia
Fecha Recepción: 24 de febrero de 2013
Fecha Aceptación: 25 de noviembre de 2013
The synthesis of faujasite type zeolite from an illite-rich raw material by an alkaline fusion step prior to hydrothermal treatment was investigated. The synthesis products were characterized by X-ray fluorescence, X-ray diffraction, scanning electron microscopy and Fourier transformed infrared spectroscopy, in order to elucidate their physicochemical and mineralogical characteristics. The transformation of the starting material can be summarized by two processes: (1) destruction of the aluminosilicate structure and (2) crystallization in a zeolite structural framework. The as-synthesized faujasite was tested as an adsorbent material in the removal of Cr3+ and Ni2+ from aqueous solutions.
Keywords: faujasite type zeolite, illite, alkaline fusion, aluminosilicate structure, adsorption.
de Cr3+ y Ni2+ de aguas residuales provenientes del galvanizado
La síntesis de zeolita tipo faujasita a partir de una materia prima rica en illita por el método de fusión alcalina previa al tratamiento hidrotermal fue investigada. Los productos sintéticos fueron caracterizados por fluorescencia de rayos X, difracción de rayos X, microscopia electrónica de barrido y espectroscopía infrarrojo por transformada de Fourier, con el fin de establecer sus características fisicoquímicas y mineralógicas. La transformación del material de partida puede resumirse a partir de dos procesos: (1) destrucción de la estructura del aluminosilicato y (2) cristalización en un arreglo estructural característico de las zeolitas. La faujasita sintetizada fue probada como material adsorbente en la remoción de Cr3+ y Ni2+ de soluciones acuosas.
Palabras clave: zeolita tipo faujasita, illita, fusión alcalina, estructura del aluminosilicato, adsorción.
Nearly all industrial effluents are contaminated with heavy metals, which are highly toxic, non-biodegradable and may be cancerogenic. The most common pollutants which are arising from industries such as electroplating, mineral processing, galvanization plants, paints formulation, porcelain enameling, nonferrous metal and vegetable fat producing industries [1]. Due to the discharge of large amounts of metal-contaminated wastewater, the electroplating industry is one of the most hazardous among the chemical-intensive industries [2]. Inorganic effluent from these industries contains toxic metals such as Cd, Cr, Cu, Hg, Ni, Pb, and Zn, which tend to accumulate in the food chain and they are usually associated with toxicity [3]. Several treatment technologies have been developed for the removal of these metals from wastewater, which include precipitation, ion exchange methods and electrolytic techniques [4,5]. Zeolites are crystalline, microporous, hydrated aluminosilicates of alkaline or alkaline earth metals. The frameworks are composed of [SiO4]4â and [AlO4]5â tetrahedra, which corner-share to form different open structures. The tetrahedra are linked together to form cages connected by pore openings of defined size; depending on the structural type, the pore sizes range from approximately 0.3-1.0nm [6]. Due to their exceptional properties, zeolites have been widely used in numerous technical applications as catalysts, adsorbents and ion exchangers [7]. The rapid increase in consumption of zeolites calls for further work seeking cheaper raw materials for their synthesis. Generally, zeolites are synthesized from freshly prepared alkali metal aluminosilicate gels using several silica and alumina sources by hydrothermal treatment. However, the preparation of synthetic zeolites from chemical sources of silica and alumina is expensive. Therefore, in order to reduce costs, zeolite researchers are seeking cheaper raw materials for zeolite synthesis, which include clay minerals such as kaolinite [7-9], halloysite [10], interstratified illite-smectite [11], montmorillonite [12] and bentonite [9,13]. Illite is a non-expanding clay mineral and forms part of phyllosilicate group. Its structure is constituted by the repetition of tetrahedron-octahedron-tetrahedron (TOT) layers. The interlayer space is mainly occupied by poorly hydrated potassium cations responsible for the absence of swelling. This natural clay is another interesting material because is inexpensive and can be incorporated into environmental management projects. However, its potential application in zeolite synthesis and water technology has not been used.
Several studies have been carried out on the use of clay minerals in zeolite synthesis for the removal of heavy metals from aqueous solution by adsorption. However, to the knowledge of the authors, the synthesis of faujasite, type zeolite, from illite and its use as an adsorbent for the removal of Cr3+ and Ni2+ from aqueous solution has not been reported elsewhere. In this paper, we report for the first time the synthesis of natural illite clay-based faujasite with potential application in Cr3+ and Ni2+ removal from aqueous solutions.
Experimental procedure and materials
The natural clay used as starting material in this work for zeolite synthesis corresponds to illite-rich clay from the Barroblanco mine, situated in the municipality of Oiba, Santander (Colombia). The raw material was prepared prior to the synthesis process by drying during 24h, and pulverized with an agate Mortar grinder RETSCH RM 100. Finally, the sample was sieved and particles of 63μm were selected for zeolite synthesis. On the other hand, illite was thermally activated at 900°C to determine the effect of the dehydroxylation process in the synthesis process. The powder samples were then ground as fine as possible by using mortar and pestle for further characterization. Activating was done using the following chemical reagents: sodium hydroxide, NaOH, as pellets (99wt%, Aldrich) and distilled water. Illite generally is unreactive in the natural form. Subsequently, it was transformed to a more reactive (amorphous) form by subjecting it to heating at 600°C before using it as a reactant. To determine the removal efficiency of Cr3+ and Ni2+ of the as-synthesized zeolite, a wastewater sample was collected from an electroplating industry located at Bucaramanga, Santander (Colombia). For quantification of the content of chromium (Cr) and nickel (Ni) in water samples contaminated with Chromium and Nickel from Business Nichrome (nickel and chrome) metropolitan area of Bucaramanga, was performed in a brand Atomic Absorption Spectrophotometer PerkinElmer which is located in Instrumental Chemistry Laboratory of the Universidad Industrial de Santander.
Synthesis of faujasite type zeolite
An alkaline fusion step was introduced prior to hydrothermal treatment, because it plays an important role in enhancing the hydrothermal conditions for zeolite synthesis. On the other hand, this approach was adopted in this study because larger amounts of aluminosilicates can be dissolved employing this method. Raw and calcined at 900°C materials were dry mixed with NaOH pellets (starting material/alkaline activator = 1/1.2 in weight) for 30min and the resultant mixture was fused at 600°C for 1h. The alkaline reagent added to the starting material acts as an activator agent during fusion. The product yield from the fusion can be as high as 100%. Some of the inert crystalline phases in the raw materials can be fully reacted. The alkaline fused product was ground in a mortar and then 4.40g of this sample was dissolved in 21.50mL of distilled water (ratio = 1/4.9) under stirring conditions for 30min and then the reaction gel was aged for 24h to form the amorphous precursors. The amount of reagents used for the preparation of the hydrogels was based on previous literature search. Crystallization was carried out by hydrothermal synthesis under static conditions in PTFE vessels of 65mL at 80°C for different reaction times (6, 24 and 96h). At the end of the process the solid is separated by filtration, washed thoroughly several times with distilled water until the filtrate pH reduced to less than 10. The precipitated solid was dried at 100°C overnight. The dried samples were weighed and kept in plastic bags for characterization.
Characterization
X-ray diffraction (XRD) patterns of illite and as-synthesized products were recorded with a RIGAKU D/MAX IIIB diffractometer operating in Bragg-Brentano geometry with Cu-α1 radiation (k = 1.5406Å) at 40kV and 20mA and graphite monochromation. The scan parameters were step size 0.02°, dwell time 12s and 2θ range 2-70°. Chemical and thermal treatments were conducted as follows: saturation with K+ and Mg2+ ions, calcinations at 350 and 550°C and solvatation with ethylenglycol. Phase identification was performed by the Hanawalt method using the crystallographic database Powder Diffraction File (PDF-2) from International Centre for Diffraction Data (ICDD). Full-pattern Rietveld refinement using RIQAS 3.1 program (MDI Inc.) was performed to quantify the amounts of phases in illite and as-synthesized products. The chemical composition of illite and as-synthesized products was investigated by X-ray fluorescence (XRF) in a Shimazu EDX 800 HS XRF spectrometer. The quantification of the elements was carried out using the method of fundamental parameters (FP) with the software DXP-700E Version 1.00 Rel. 014. The morphology of the raw and modified clay was examined by environmental scanning electron microscopy (ESEM) (FEI Quanta 200), under the following analytical conditions: magnification = 2500-20000x, WD = 9.4, HV = 7.0kV, spot = 3.0, mode SE, detector LFD. The frameworks of the illite and its derivate products were confirmed by Fourier transformed infrared (FT-IR) spectroscopy by using a Bruker FT-IR Tensor 27 Spectrometer in the 4000-400cm-1 region. Approximately 2mg of the sample plus 200mg of KBr were weighted out, milled and grounded in a mortar. The resulting mixture was then pressed into a pellet.
Determination of Cr3+ and Ni2+ by atomic ab-sorption spectrometry
A calibration curve is a general method for de-termining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. Synthetic monoionic solutions with concentrations of 1000mg/L of Cr3+ and Ni2+ were used as stan-dards in order to prepare calibration curves for the-se metal ions. One aliquot of 10mL of the standard solutions was initially taken and then it was diluted to 100mL with distilled water in a 250mL Erlen-meyer flask. The final concentration was obtained through the equation:
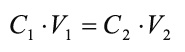
where C1 is the initial concentration of each heavy metal, V1 is the initial volume of the aliquot, C2 is the final concentration of each heavy metal, and V2 is the final volume. Therefore, the final concentration of Cr3+ and Ni2+ was 100mg/L. The preparation of the calibration curves from the standards within these metal ions ranges was carried out using the final concentration of 100mg/L calculated above. Eleven aliquots of 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5.0mL of the standard solutions were taken to prepare the calibration curves for Cr3+ and Ni2+. 0.5mL of nitric acid (HNO3) these solutions were first spiked to an Erlenmeyer flask, then the calculated amount of volume of the standard solutions were added. Finally, the resultant solutions were diluted to 100mL with distilled water. The final concentration of each solution was determined by the above equation and the results were tabulated. Using the technique of atomic absorption spectrometry absorbance of each pattern (Perkin-Elmer 372 atomic absorption spectrophotometer), where the calibration curve by linear regression method to calculate the concentrations of Cr3+ and Ni2+ in electroplating wastewater was determined.
Adsorption experiments in batch mode
The sorption of Cr3+ and Ni2+ onto illite-based faujasite type zeolite was studied in laboratory batch experiments, which were carried out at room temperature to investigate the efficiency of this adsorbents material for removing heavy metals from aqueous solution. A weighed amount of sorbent (0.25 and 0.5g) was introduced in 180g amber glass bottles, and then a volume of 50mL of electroplating industry wastewater was added. Later, the sorbent: aqueous solution mixtures were continuously agitated in a Shaker BIOT-S-04 (GFL) for 24h, and the temporal evolution of the pH of the solution and electrical conductivity (EC) was monitored. At each scheduled reaction time (0, 5, 10, 15, 30, 45, 60, 120, 240, 360, 720 and 1440min) the bottles were removed from the shaker and the adsorbents were separated by filtration, while the filtrates were stored in a refrigerator for chemical analyses. All measurements were done according to the Standard Methods for the Examination of Water and Wastewater. Both pH and EC of the original and treated aqueous solutions were measured using a pH Meter Lab 870 (Schott Instruments) and a 712 conductometer (Metrohm AG), respectively. The efficiency of treatment of the electroplating effluent using faujasite was then determined by the following equation:

Where C1= initial metal concentration and C2= metal concentration after treatment.
The sample has a chromium concentration of 117.3mg/L and a nickel concentration of 132.3mg/L.
Characterization of the starting material
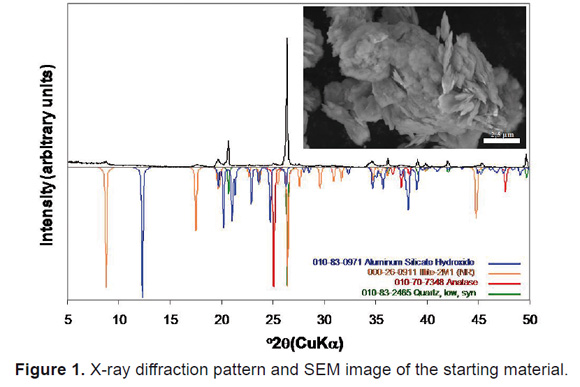
The chemical composition of illite is 63.02wt% SiO2, 29.08wt% Al2O3, 3.54wt% K2O, 1.43wt% TiO2, 1.20wt% Fe2O3, 1.11wt% MgO, 0.22wt% CaO, and 0.16wt% Na2O [7]. As shown in the XRD pattern of the Figure 1, illite is the predominant mineral phase in the starting material and is identified by a series of basal reflections at 10.1Å, 4.98-5.01Å, 3.33Å, and 2.89-2.92Å. However, minor impurities, such as quartz, anatase and aluminum silicate hydroxide also occur. Quartz is identified by its distinctive reflections at 4.26 and 3.35Å. The 3.35Å peak of quartz was more intense than the other peaks. Illite can be recognized by its sheets or large flacky crystals, which develop clusters (Figure 1).
Characterization of the zeolitic materials
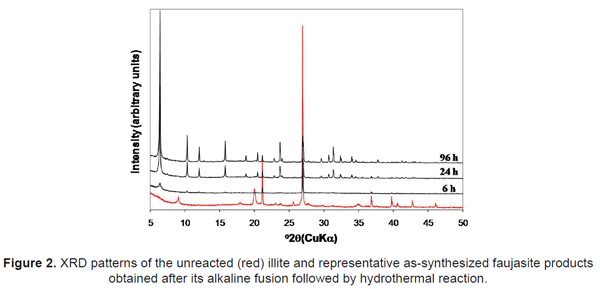
XRD patterns in Figure 2 show the progress of faujasite synthesis by the fusion method, revealing the reduction of intensity of the characteristic peaks of the starting material (illite) and the appearance of new reflection peaks corresponding to zeolitic materials, which show a progressive increase in the intensity with reaction time. After 6h of reaction faujasite peaks appear, with a maximum crystallinity occurring after 96h. Therefore, the reaction time influences the crystallinity of the synthesis products. The synthesis of zeolite via alkaline fusion followed by hydrothermal treatment reveals that the alkaline fusion process promoted the dry reaction between the crystalline mineral phases present in illite and the alkaline activator, and the alkaline fused product corresponds to amorphous sodium aluminosilicate, indicating that fusion was very effective in extracting the silicon and aluminium species from illite.
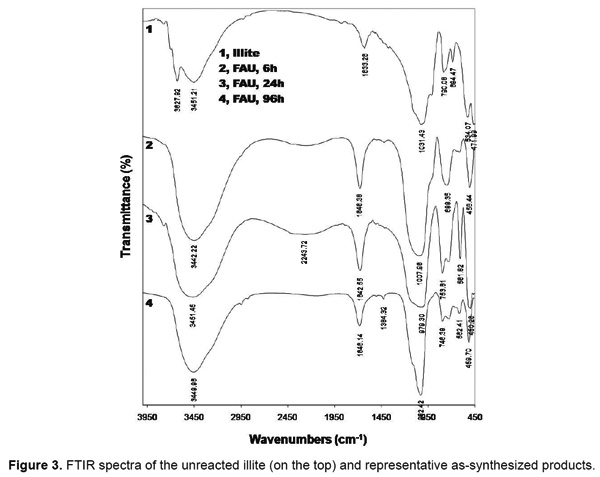
The presence of aluminosilicate framework in the faujasite structure was confirmed using FT-IR spectroscopic technique. Infrared spectra of the untreated illite and zeolitic products are shown in Figure 3. After 6h, there is appearance of absorbance band situated around at 560cm-1, characteristic of zeolites with double ring and specifying the presence of the zeolites such as faujasite as revealed by Gruj'c E, et al. [14]. Moreover, the observed single strong band at 3450cm-1 ascribed to the presence of hydroxyls in the faujasite supercages and sodalite cages as the building blocks of faujasite structures [15].
New infrared bands located towards 700 and 750cm-1 appears after 24h of reaction, although they may reveal the presence of hydroxysodalite [14], which is a zeolitic phase that can be formed at expenses of faujasite. All these observations confirm the formation of faujasite-type zeolite after alkali fusion followed by hydrothermal treatments of natural clay. We observed also that bands vibration characteristic of illite do not appear in the final products, whereas quartz present in the end product is characterized by the bands of vibrations appearing at 700, 780 and 800cm-1 are associated with T-O (T = Al, Si) symmetric stretching vibrations. Infrared spectral results are in good agreement with XRD results.
Conditions wastewater
The initial concentrations of wastewater contaminated with ions Cr3+ and Ni2+ in addition to its pH and electrical conductivity were measured. These yielded values of 117.30mg/L Cr+3, with a pH of 3.2 and an electrical conductivity of 43.24mS/cm. Wastewater from Ni2+ ion showed a concentration of 132300mg/L, a pH of 6.75 and an electrical conductivity of 142.80S/cm. Taking into account that the industrial water used to carry out the removal of Cr+3, Ni+2 ions and not only an effluent contaminated with these elements, also has some other ions such as iron, copper, cadmium, zinc, lead in smaller proportions. These ions may depicting competition of ions at the time of the adsorption capacity of the zeolite, and this results in reducing the efficiency of the removal of ions by said zeolite. They may have an association with the ionic competition for samples of nickel and chromium in which many of the ions in solution, their behavior tend to stay within the zeolite, implying that some of the ions are not eliminated completely and remain in solution.
Adsorption tests
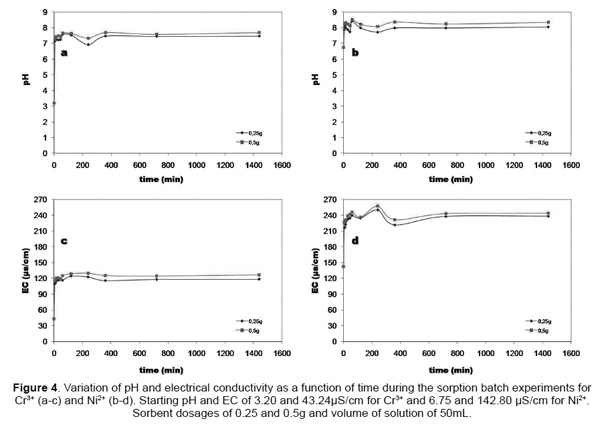
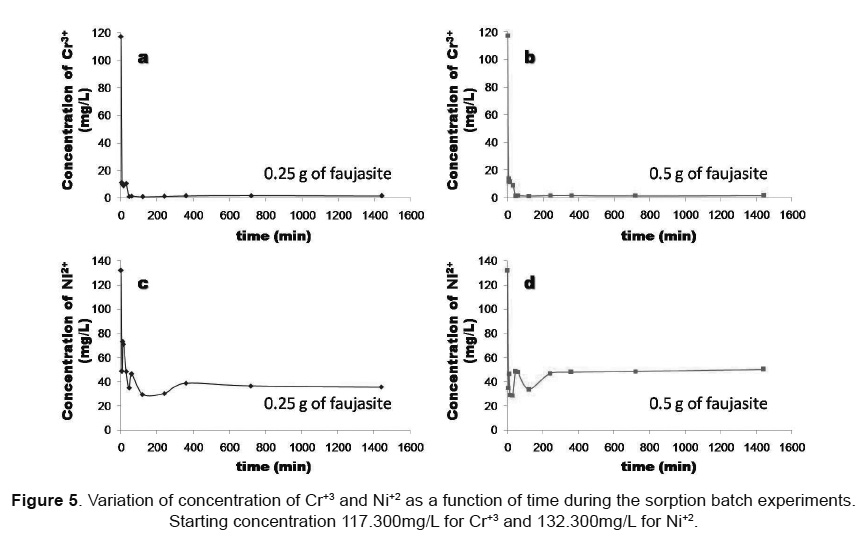
Kinetics of the neutralization reaction. Neutraliza-tion is generally the first step in the treatment of the electroplating solution containing Cr3+ and Ni2+. The-refore, the kinetics of the neutralization reaction was investigated by monitoring the pH and EC of faujasi-te/ aqueous solution mixtures (0.25g/50mL and 0.50 g/50mL) over a period of 24h. The effect of contact time on pH and EC during the batch experiments for Cr3+ and Ni2+ is shown in Figure 5. Results indicate that Cr3+ and Ni2+ adsorption by faujasite was highly pH-dependent and increased with increasing pH conditions. pH increased rapidly within the first 5min of contact between the solution and the sorbent (illite-based faujasite), and then it stabilized (Figures 4a and 4b). According to Genç-Fuhrman et al. [16], pH increases mainly due to dissolution of the sorbent in the process of shaking. Final pH values of 7.58-7.69 for Cr3+ and 8.42-8.53 for Ni2+ were observed in the batches due to hydrolysis of the faujasite as well as cationic exchange. Similar results are repor-ted elsewhere with a remark that the pH increase is almost unavoidable in a zeolite heavy metal system [17]. On the other hand, results reveal that the in-crease of the adsorbent dosage promoted higher pH conditions. A similar behavior was observed for EC as shown in Figures 4c and 4d.
Removal of Cr3+ and Ni2+
The immobilization of heavy metal ions from aqueous solutions is quite a complicated process, consisting of ion exchange and adsorption and is likely to be accompanied by precipitation of metal hydroxide complexes on active sites of the particle surface [18]. The kinetics of the Cr3+ and Ni2+ adsorption was also studied over 24h. Metal removal trends as a function of contact time after batch reaction are illustrated in Figure 5. Results indicate that faujasite produced a steep decrease in Cr+3 concentration within the first 5min, reaching very low residual concentrations. However, after 45min plateau values were reached for the rest of the time intervals, indicating a complete removal. Cr shows an abrupt decrease in concentration from 0 to 45min and tends to stabilize at values between 0.922 and 1.695mg/L (0.25g of zeolite) and between 0.946 and 1.513mg/L (0.5g of zeolite). Ni showed an inconsistent variation of concentration between 0 and 360min, which is revealed by the fluctuations observed during the batch experiments, and it tends to stabilize at values between 35.6 and 38.7mg/L (0.25g of zeolite) and between 47.9 and 50.1mg/L (0.5g of zeolite).
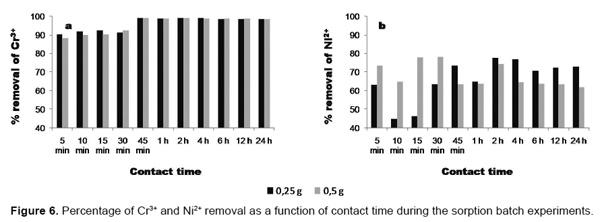
As shown in Figure 6, the removal efficiency of metal ions by faujasite produced the following ranges: Cr3+ (90.53- 99.21% and 88.31 - 99.19%, using 0.25 and 0.5g of zeolite, respectively) and Ni2+ (44.74 - 77.73% and 62.07 - 78.30%, using 0.25 and 0.5g of zeolite, respectively). Sorption tests reveal that both metal ions were rapidly removed by faujasite within 45min (Cr3+) and 360min (Ni2+) with 88.31 - 90.53% and 63.24 - 73.54% of the metal removal achieved in the first 5min for Cr3+ and Ni2+, respectively. However, sorbent produced lower Ni2+ removal (88.31-99.21%) compared with that for Cr3+ (44.74 - 78.30%). Therefore, the competition for sorbent adsorption sites in the presence of Cr3+ produced a decrease in the uptake of Ni2+. No significant adsorption was observed after 45min (Cr3+) and 360min (Ni2+) of contact time. According to Peric et al. [18], the immobilization of heavy metals from aqueous media is a complex process, which consists of ion exchange and adsorption and is likely to be accompanied by precipitation of metal hydroxide complexes on active sites of the particle surface. On the other hand, the addition of an alkaline material such as faujasite to the electroplating wastewater increased the pH (7.58-7.69 for Cr3+ and 8.42-8.53 for Ni2+) and these metal ions could be hydrolyzed and precipitated as suggested by Evangelou and Zhang [19]. However, the efficiency of the tested sorbent with respect to metal retention and/or metal concentration control during its application for the treatment of metal-bearing aqueous media is governed by parameters like contact time, pH, temperature and sorbent nature as demonstrated in previous studies [20]. On the other hand, mechanisms of interactions, such as precipitation and adsorption, between Cr3+ and Ni2+ and faujasite are strongly influenced by pH.
Effect of sorbent dosage
The adsorption of Cr3+ and Ni2+ was studied by increasing the adsorbent dosage from 0.25 to 0.5g/50mL. The adsorption efficiency generally improved with increasing adsorbent dosage up to a certain value and then remained constant. The increase in the adsorption percentage with increasing adsorbent dosage is due to the increase in the number of adsorbent sites [21]. However, this does not indicate that the amount removed is directly proportional to the amount of absorbent used. Sorption test results reveal that residual concentrations of Cr3+ obtained using 0.25g of faujasite are a little bit higher compared with those observed with a higher sorbent dose (0.5g). However, higher residual concentrations of Ni2+ were obtained when a higher sorbent dose was used.
Effect of contact time
As shown above, both metal ions were very rapidly removed by faujasite in the first 5min. The adsorbed amount of metal ions increased from 5 to 45min for Cr3+ and from 5 to 360min for Ni2+. Then adsorption rate gradually decreases and removal reaches equilibrium. The time required to reach equilibrium was dependent on metal ion.
Adsorption kinetics
Previous studies [22-28] reveal the effect of ion competition on metal uptake from electroplating wastewater. Given that this effluent is not only contaminated with Cr3+ and Ni2+ but also with other ions such as iron, copper, cadmium, zinc, lead, in minor proportions, which may represent ion competition at the time of adsorption by the zeolite, this results in lower removal efficiency of Cr3+ and Ni2+ with many of the ions in solution showing a behavior that tends to stay within the adsorbent thus implying that some of the ions to be removed are not removed in its entirely and remain in solution. The adsorption of Cr3+ and Ni2+ by faujasite was strongly affected by pH of the aqueous solution, which is an important controlling parameter in the adsorption process [29-30]. At lower pH value, the H+ ions compete with metal cations for the exchange sites in the system thereby partially releasing the latter [25,27]. The maximum adsorption of Cr3+ and Ni2+ was probably carried out at a pH greater than 6 to avoid competition between ions mentioned above, which can lead to a minimal adsorption at a pH around 2, increasing the mobility of ions into an adsorption of H+ [25]. The pH increase is mainly due to dissolution of the inorganic absorbent (faujasite) during the agitation process. The sorption trends were attributed to the competition between Cr3+ and Ni2+ and proton for the binding sites on faujasite surface. At low pH, an excess proton can compete with the metal ions, resulting in a low level of metal ion adsorbed. The pH-dependence of adsorption suggests that Cr3+ ions are adsorbed according to the ion-exchange mechanism as suggested by Rengaraj et al. [31]. However, precipitate could not be excluded at a higher pH [30]. The lower affinity for Ni2+ can be explained by its high binding capacity for soluble ligands or its poor competitiveness with other metals in the same solution [22]. Most studies on kinetic process of metal ion adsorption, a distinct two-step behavior was reported [26,32], which can explain the metal ion trends observed for Cr3+ and Ni2+ after treatment of the electroplating wastewater. Liu and Huang [32] attributed the two-step adsorption characteristic to the heterogeneity of the surface binding sites on sorbents, explaining that different binding sites had different binding affinities to metal ions and resulted in different binding rates. However, Qin et al. [26] asserted that the fast initial sorption owed to the fast transfer of metal ions to the surface of sorbent particles, while the following slow sorption was as a result of the slow diffusion of metal ions into the intra-particle pores of sorbents.
The natural clay, in which illite coexists with quartz was transformed in faujasite type zeolite by fusion with NaOH powder followed by hydrothermal treatment. This synthesis method produced a highly crystalline faujasite type zeolite, obtained after short times of crystallization. This study shows that faujasite can assist in Cr3+ and Ni2+ pollution control. Findings of our research indicate that Cr3+ removal can be much better accomplished by faujasite compared to Ni2+ removal during the treatment of electroplating or other industrial effluents. This research can be also used as a reference for future in depth studies considering alternative technologies applied to the mitigation of the environmental impact produced by the electroplating industry.
This research forms part of the undergraduate thesis of V. Contreras and J. Castrillón and has benefited from research facilities provided by the Universidad Industrial de Santander, the Centro de Desarrollo Productivo de Joyería de Bucaramanga and the Instituto Zuliano de Investigaciones Tecnológicas. We thank to Andelfo Pinilla, Mario Macías and Eric Plaza for assistance with XRD, XRF and SEM data acquisition.
[1] Meena AK, Mishra GK, Rai PK, Rajagopal Ch, Nagar PN. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005;122(1-2):161-70. [ Links ]
[2] Pereira FV, Alves-Gurgel, LV, Gil, LF. Removal of Zn2+ from aqueous single metal solutions and electroplating wastewater with wood sawdust and sugarcane bagasse modified with EDTA dianhydride (EDTAD). J. Hazard. Mater. 2010;176(1-3):856-63. [ Links ]
[3] Kurniawan TA, Chan GYS, Lo WH, Babel S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006;118(1-2):83-98. [ Links ]
[4] Algarra M, Jiménez MV, Rodríguez-Castellón E, Jiménez-López A, Jiménez-Jiménez J. Heavy metals removal from electroplating wastewater by aminopropyl-Si MCM-41. Chemosphere. 2005;59(6):779-86. [ Links ]
[5] Sousa FW, Sousa MJ, Oliveira IRN, Oliveira AG, Cavalcante RM, Fechine PBA, et al. Evaluation of a low-cost adsorbent for removal of toxic metal ions from wastewater of an electroplating factory. J. Environ. Manage. 2009;90(11):3340-4. [ Links ]
[6] Szostak R. Molecular Sieves, Principles of Synthesis and Identification. New York: Van Nostrand Reinhold; 1989. [ Links ]
[7] Breck DW. Zeolite Molecular Sieves: Structure, Chemistry and Use. New York: John Wiley; 1974. [ Links ]
[8] Barrer RM. Zeolites and Clay Minerals as Sorbents and Molecular Sieves. London: Academic Press; 1978. [ Links ]
[9] Boukadir D, Bettahar N, Derriche Z. Synthesis of zeolites 4A and HS from natural materials. Ann. Chim. Sci. Mat. 2002;27(4):1-13. [ Links ]
[10] Klimkiewicz R, Drąg EB. Catalytic activity of carbonaceous deposits in zeolite from halloysite in alcohol conversions. J. Phys. Chem. Solids. 2004;65(2-3):459-64. [ Links ]
[11] Baccouche A, Srasra E, Maaoui ME. Preparation of Na-P1 and sodalite octahydrate zeolites from interstratified illite-smectite. Appl. Clay Sci. 1998;13(4):255-73. [ Links ]
[12] Cañizares P, Durán A, Dorado F, Carmona M. The role of sodium montmorillonite on bounded zeolite-type catalysts. Appl. Clay Sci. 2000;16(5-6):273-87. [ Links ]
[13] Ruiz R, Blanco C, Pesquera C, González F, Benito I, López JL. Zeolitization of a bentonite and its application to the removal of ammonium ion from waste water. Appl. Clay Sci. 1997;12(1-2):73-83. [ Links ]
[14] Grujić E, Subotić B, Despotović LJA. Transformation of Zeolite A Into Hydroxysodalite. III. The Influence of Temperature on The Kinetics of Transformation. In: PA Jacobs and RA van Santen, Editors, 585 Zeolites: Facts, Figures, Future, Studies in Surface Science and Catalysis 49, Elsevier, 586 Amsterdam 1989. p. 261-70. [ Links ]
[15] Rujiwatra A. A selective preparation of phillipsite and sodalite from perlite. Mat. Lett. 2004;58(14):2012-5. [ Links ]
[16] Genç-Fuhrman H, Mikkelsen PS, Ledin A. Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater: Experimental comparison of 11 different sorbents. Water Res. 2007;41(3):591-602. [ Links ]
[17] Pitcher SK, Slade RCT, Wards NI. Heavy metal removal from motorway stormwater using zeolites. Sci. Total Environ. 2004;334-335:161-6. [ Links ]
[18] Peric J, Trigo M, Medvidovi'c NV. Removal of zinc, copper and lead by natural zeolite-a comparison of adsorption isotherms. Water Res. 2004;38(7):1893-9. [ Links ]
[19] Evangelou VP, Zhang YL. A review: Pyrite oxidation mechanisms and acid mine drainage prevention. Crit. Rev. Env. Sci. Tec. 1995;25(2):141-99. [ Links ]
[20] Hequet V, Ricou P, Lecuyer I, LeCloirec P. Removal of Cu2+ and Zn2+ from aqueous solutions by sorption onto mixed fly ash. Fuel. 2000;80(6):851-6. [ Links ]
[21] Selvaraj K, Manonmani S, Pattabhi S. Removal of hexavalent chromium using distillery sludge. Bioresource Technol. 2003;89(2):207-11. [ Links ]
[22] Sterritt RM, Melanie JB, Lester JN. Metal removal by adsorption and precipitation in the activated sludge process. Environ. Pollut. 1981;24(4):313-23. [ Links ]
[23] Henderson RW, Lightsey ER, Poonawala NA. (1977). Competitive adsorption of metal ions from solutions by low-cost organic materials. B. Environ. Contam. Tox. 1997;18(3):340-4. [ Links ]
[24] Low KS, Lee CK, Leo AC. Removal of metals from electroplating wastes using banana pith. Bioresource Technol. 1995;51(2):227-31. [ Links ]
[25] Ajmal M, Rao RA, Ahmad R, Ahmad J. Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni(II) from electroplating wastewater. J. Hazard. Mater. 2000;79(1-2):117-31. [ Links ]
[26] Qin F, Wen B, Shan X, Xie Y, Liu T, Zhang S, Khan S. Mechanisms of competitive adsorption of Pb, Cu, and Cd on peat. Environ. Pollut. 2006;144(2):669-80. [ Links ]
[27] Wang XS, Qin Y. Removal of Ni(II), Zn(II) and Cr(VI) from aqueous solution by Alternanthera philoxeroides biomass. J. Hazard. Mater. 2006;138(1):582-8. [ Links ]
[28] Suksabye P, Thiravetyan P, Nakbanpote W, Chayabutra S. Chromium removal from electroplating wastewater by coir pith. J. Hazard. Mater. 2007;141(3):637-44. [ Links ]
[29] Elliott HA, Huang CP. The Adsorption of Cu(II) Complexes onto Aluminosilicates. Water Res. 1981;15(7):849-55. [ Links ]
[30] Wu Y, Zhang S, Guo X, Huang H. Adsorption of chromium(III) on lignin. Bioresource Technol. 2008;99(16):7709-15. [ Links ]
[31] Rengaraj S, Joo CK, Kim Y, Yi J. Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins 1200H, 1500H and IRN97H. J. Hazard. Mater. 2003;102(2-3):257-75. [ Links ]
[32] Liu C, Huang PM. Kinetics of phosphate adsorption on iron oxides formed under influence of citrate. Soil Sci. 2000;80(3):445-54. [ Links ]