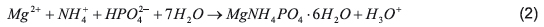Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista ION
Print version ISSN 0120-100X
Rev. ion vol.29 no.1 Bucaramanga Jan./June 2016
https://doi.org/10.18273/revion.v29n1-2016001
DOI: http://dx.doi.org/10.18273/revion.v29n1-2016001
digestion systems: An overview
Maycoll Stiven Romero-Güiza1,2,3*; Joan Mata-Alvarez1; Josep María Chimenos Rivera2;
Sergi Astals Garcia1,4
1 Department of Chemical Engineering, University of Barcelona, Martí i Franquès, 1, 08028 Barcelona, Spain.
2 Department of Materials Science and Metallurgical Engineering, University of Barcelona, Martí i Franquès, 1, 08028 Barcelona, Spain.
3 IRTA, GIRO Joint Research Unit IRTA-UPC, Torre Marimon, 08140 Caldes de Montbui, Barcelona, Spain.
4 Advanced Water Management Centre, The University of Queensland, St Lucia, QLD 4072, Australia.
Fecha Recepción: 3 de junio de 2015
Fecha Aceptación: 17 de diciembre de 2015
Anaerobic digestion is a worldwide technology to treat organic waste streams, primarily due to its capacity to produce methane as renewable energy. However, there is an increasing interest on nutrient recovery (N and P), which from both environmental and economic reasons have been identified as key feature in anaerobic digestion plants. The manuscript presents a comprehensive overview on recent advances in nutrient recovery technologies applicable for anaerobic digestion systems. The review focus on N and P recovery through the use of digestates as fertilizers, struvite precipitation and biological systems such as phycoremediation (i.e. algae cultivation) and polyphosphates accumulating organisms.
Keywords: Anaerobic digestion, nutrient recovery, struvite, phycoremediation, fertilizer.
sistemas de digestión anaeróbica: revisión
La digestión anaeróbica es una tecnología mundialmente aplicada para el tratamiento de residuos orgánicos, principalmente debido a su capacidad de producir metano como fuente de energía renovable. Sin embargo, existe un creciente interés en la recuperación de nutrientes (N y P), el cual desde el punto de vista ambiental y económico ha sido identificado como un factor clave en las plantas de tratamiento vía digestión anaeróbica. El presente manuscrito presenta una visión global de los recientes avances en las tecnologías de recuperación de nutrientes aplicables a sistemas de digestión anaeróbica. La revisión está enfocada en la recuperación de N y P mediante el uso del digestado como fertilizante, la precipitación de estruvita y sistemas biológicos como la ficoremediación (ej. cultivos de algas) y organismos acumuladores de polifosfatos.
Palabras clave: Digestión anaeróbica, recuperación de nutrientes, estruvita, ficoremediación, fertilizante orgánico.
sistemas de digestão anaeróbia: revisão
A digestão anaeróbia é uma tecnologia mundialmente conhecida para o tratamento de resíduos orgânicos, principalmente devido a sua capacidade de produzir metano como energia renovável. No entanto, há um interesse crescente sobre a recuperação de nutrientes (N e P), que a partir de razões ambientais e econômicas têm sido identificados como elemento-chave em plantas de digestão anaeróbia. O presente manuscrito apresenta uma visão abrangente sobre os recentes avanços em tecnologias de recuperação de nutrientes aplicáveis para sistemas de digestão anaeróbia. O foco da revisão é a recuperação do N e P através do uso de fertilizantes, como digestates precipitação estruvite e sistemas biológicos, tais como phycoremediation (por ex, cultivo de algas) e organismos acumuladores de polifosfatos.
Palabras-chave: digestão anaeróbia, recuperação de nutrientes, estruvite, fitorremediação, adubo orgânico.
Cita: Romero-Güiza MS, Mata-Alvarez J, Chimenos Rivera JM, Astals Garcia S. Nutrient recovery technologies for anaerobic digestion systems: An overview. rev.ion. 2016;29(1):7-26.
Anaerobic digestion (AD) stands as an important technology in the emerging green energy economy [1]. Advantages of AD over other technologies are: (i) cost-effective organic wastes treatment for wastewater and solid waste streams, and (ii) energy/economy alternative in rural sector through the digestion of agro-wastes and/or energy crops [2,3]. AD plants are usually of large scale, with digester capacities ranging from few hundred m3 up to several thousand m3 [4]. In urban areas AD plants mostly treat sewage sludge and organic fraction of municipal solid wastes (OFMSW) [5,6]; while, rural biogas plants, centralized and on-farm, co-treat animal manure and other suitable organic residues [3]. Centralized plants commonly develops in high density of livestock areas linked to insufficient accessible arable land (i.e. Europe) [7,8]; while on-farm plants are becoming for interest on extensive regions with biogas incentives (i.e. United States and Australia) [9]. However, the uses of house digester have started to rise in the developing countries as energy/biogas source. On biogas plants the biogas can, after cleaning, produce heat in a quality adapted burner, or electricity and heat in a combined heat and power unit [8]. However, biogas can also be transformed into green gas by upgrading which has the same quality as natural gas and can be supplied to the natural-gas grid or used as transport fuel [10].
Besides renewable energy, AD of organic wastes produces a digestate which is a mixture of partially degraded organic matter, anaerobic biomass and inorganic matter (including nutrients) [11]. The AD process facilitates the mobilization of nutrients (N and P) from the organic matter to the liquid phase. N is converted into ammonium and organic P is hydrolyzed to soluble P [12,13]. Digestate quality and mobilization extent depend on the three main components of the anaerobic digestion system: (i) the feedstock, (ii) the digester design and operational conditions, and (iii) digestate post-treatment [14]. As an example, Figure 1 illustrates the most common organic fraction municipal solid waste (OFMSW) AD plant configurations.
Today, most AD plants are energy focused with low attention on nutrient recovery. Nevertheless, the continuous increase in fertilizer prices (mainly formed of N, P and K) has raised interest on nutrient recovery from digestate. Batstone and Virdis [15] clearly stated that new wastewater treatment plants have to: (i) achieve existing public health and environmental goals, (ii) recover maximal energy from wastewater, and (iii) preserve and recover nutrients for reuse. Moreover, the economic success of investment in AD plants is strictly related to incentive polices adopted in the countries. Actually, a high proportion of AD operators seldom sell digestates above cost recovery prices, despite its high agronomic value [9]. Therefore it has been provide interest and incentives to nutrient recovery from AD effluents. For instance, biogas Italy subsidies provide a plus an added bonus (15-30€/MWh) if nitrogen is removed to produce a fertilizer [16,17].
Digestate direct land application
Today, using digestate as organic fertilizer or soil conditioner seem to be the best option for its recycling [3,13,18]. The use of digestate as fertilizer allow to recycle nutrients and reduce the use of chemical fertilizers [19]. Nonetheless, the quality of the digestate must be carefully evaluated prior usage [18].
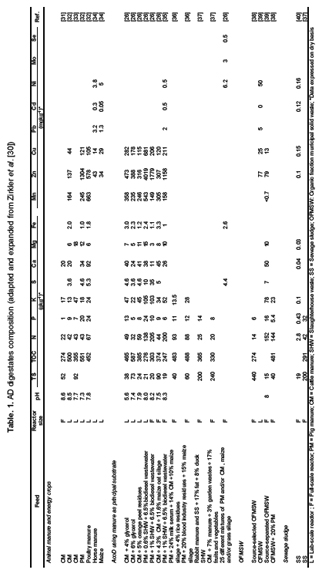
High quality digestate fit for use as fertilizer is defined by essential features such as declared content of nutrients, pH, dry matter and organic dry matter content, homogeneity, purity (free of inorganic impurities such as plastic, stones, glass, etc), content of biological (pathogenic) material and of chemical pollutants (organic and inorganic) [20]. From an agricultural point of view, the main parameters to take into consideration are pH, salinity, nutrients, pathogens and heavy metals [3,13,21], while environmental concerns are inappropriate digestate handling, storage and application, which may led to ammonia emissions, nitrate leaching and phosphorous overdoses [22]. Table 1 shows the heavy metals, micro- and macro-nutrients composition of different AD digestate, which have been grouped in five categories [3,23]: (i) sewage sludge (SS); (ii) animal manures; (iii) food industry wastes; (iv) energy crops and harvesting residues; and (v) OFMSW. It can observe that element concentrations are highly variable and substrate type dependent. For instance, sewage sludge digestate present a relatively high P concentration while slaughterhouse waste digestate present high N concentration. Digestate are also evaluated in terms of salinity, remaining biodegradable organic matter, phytotoxicity and pathogens abundance [24,25]. Such facts determine the need for applying additional specific treatment to increase digestate quality until acceptable levels [26].
The risk of inappropriate use of digestate is related with the salinity (i.e. Na+ and Al3+) and digestate stability. High doses or continued application of high salinity digestate can lead to an excessive salt and heavy metals accumulation in soil, which might inhibit plant growth [3,26,27]; while application of unstable digestate (i.e. digestate with large amounts of non-degraded organic matter) may exert negative impact on organic matter mineralization and nutrient turn-over in the plant-soil system [13,27,28]. Unstable digestate can be obtained from badly operated digesters or digesters operated at intense conditions such as short hydraulic retention times, high organic loading rates and co-substrate addition based on random or heuristic decisions [3]. Enlarging digestion time can decrease the amount of labile compounds in digestates; however it may reduce the specific volumetric biogas production of digesters as well as require a higher investment (larger vessel). Aerobic post-treatment can be used to decrease digestates phytotoxic impact without affecting AD feasibility as well as improve pathogens destruction. In this regard, Abdullahi et al. [28] found that the seed germination increased with dilution and incubation time, suggesting that lower application rates and longer lag periods between application of aerobically treated digestate and planting can reduce the occurrence of phytotoxicity. Abubaker et al. [29], who studied bacterial community structure and microbial activity in different soils amended with digestate and untreated cattle slurry, observed that differences in microbial community structure induced by the digestate appeared to be smaller than those induced by cattle slurry, and those changes did not translate into altered microbial functioning.
Digestate Solid-Liquid separation
Although digestate direct land application is widely applied, the large amount of digestate produced in intensive livestock regions can led to nutrient surplus problems [40-42]. Additionally, the large amount of water (>80%) in digestates will increase transport expenses and difficult its utilization.
To avoid negative impacts on the environment, complementary treatment of digestate are needed in context of nutrient surplus removal and/or recovery [43,44]. One of the simplest digestate post-treatment methods is the separation of the solid and the liquid fraction (known as digestate dewatering) [45,46].
The solid-liquid separation provides two materials fractions that can be handling independently [47]. The solid fraction can be transported longer distances because of the reduction in the water content, or undergo further processing to produce value-add products (e.g. compost and organic fertilizer by bio-stabilization) [47]. The liquid fraction can be returned to the process, treated to meet lands requirements (nutrient removal), or treated to recover valuable nutrients (P and N) [48-53]. The aims of solid and liquid separation are: (i) increase the possibilities of digestate management as by-product; (ii) avoid uncontrolled decomposition process; and (iii) reduce transport cost (solid fraction) [54]. The common solid and liquid separation technologies are decanting, centrifuge, brusch-roller, vibrating screen, screw press, belt press and run-down screen [55]. The kind of technology used in the solid-liquid separation of the digestate will determine the composition of the fractions. Moreover, the separation efficiency is determined by the flows, solid content, use of additives and digestate degradation [45,53,54,56].
For instance, in manure centrifuged digestate, the dry matter content of the solid fraction is typically 25-35%, containing 60-80% of the digestate dry matter and almost the totally of phosphorus from the original slurry, but only 20 - 25% on the nitrogen and 10-15% of the potassium [14]. Another factor to consider in the solid-liquid separation is the economic efficiency which is mainly determined by the organic matter and nutrient (N, P and K) retention in the solid fraction [53].
Therefore, some processes integrate two sequential separation technologies to increase separation efficiency and provide different: (i) operation conditions (flows), (ii) performances (N and P recovery) and (iii) expenses (investment and operation cost) [57].
For instance, Parera et al. [58] evaluated the economic viability of two solid and liquid separation systems, run down screen-screw press and screw press-centrifuge; concluding that operational conditions regulation and calibration (i.e. flows and mesh pore diameter) determinate the separation efficiency. The run down screen-screw press system allowed high flow (20m3/h) with N and P recovery of 15 and 20%, respectively, while screw press-centrifuge systems worked at low flow (4.5m3/h) with N and P recovery of 45-80%, respectively. However, the economic balance showed that run-down screen-screw press system presented lower operation cost (1.03€/m3; 1.96€/kg N and 4.96€/kg P) than the screw press-centrifuge systems (3.68€/m3; 2.34€/kg N and 4.43€/kg P) [58].
Digestate solid fraction treatment
From the solid-liquid separation, the solid fraction can subsequently be applied directly as fertilizer in agriculture, composted or dried for intermediate storage and enhanced transportability. The solid fraction can be also be sold as a phosphorous rich fertilizer, without any further treatment [20]. Nest et al. [59] showed that the use of solid fraction of separated digestate may replace mineral fertilizer and leads to enhanced the availability of P. Digestate composting and stockpiling are widely used, since these technologies are the simplest techniques and the material can be considered hygienically safe [60].
On the other hand, pelletized technology is rapidly expanding. Pelletized technology consists of digestate dried (e.i. fluidized bed dryer) following by mixing with a (NH4)2SO4 solution and pellets formation [43,61]. It has been shown that when digestate is converted to dry pelletized bagged products, digestate can reach a far greater price up €250/ton [60]. Other options for digestate solid fraction is the use for industrial purposes, this involves production of composite materials, biorefinery processes or incineration for energy production [20]. Santi et al. [62] showed that digestate solid fraction produced by commercial corn-silage AD contains a notable quantity of cell wall polymers that could potentially be used in biorefinery processes for ethanol and xylo-oligosaccharide production.
Digestate liquid fraction treatment
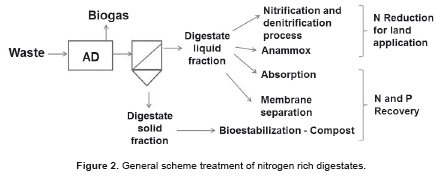
From the solid-liquid separation the liquid fraction generates greater interest, since it contains most nitrogen and potassium. Digestate liquid fraction can be used to dilute high solid feedstock and re-fed to the digester and/or applied as irrigation water [48,63]. However, the accumulation of nitrogen due to liquid recirculation can lead to anaerobic biomass inhibition [64-66] and restricts land application directives limiting N spreading on lands [67,68]. Therefore, AD plants are currently focusing their efforts on providing adequate technologies to process digestate liquid fraction, giving strategic importance to reduce nitrogen content by either removal or recovery technologies (Figure 2) [14]. Removal consist in reduce the nitrogen concentration on digestate, converting the ammonium into nitrogen gas, enhancing the nitrogen concentrations to directive limits or land requirements; while recovery consist in ammonium fixation and concentration on liquid or solid medium that are potentially reusable as agricultural fertilizer or chemical reagent [15]. Today, nitrogen treatment technologies are mainly focus on its elimination rather than on its recovery. However, conventional nitrogen removal methods are being recognised as wasteful [15,63]. For instance, the conventional nitrification-denitrification process, where nitrogen is converted to elemental nitrogen gas requires both electrical and chemical energy [15]. In this regard, new technologies such as Anammox, absorption and membrane have been presented as alternative nitrogen treatment options. A brief description of the main technologies is discussed below.
Anammox
Anaerobic ammonium oxidation (Anammox) is growing on importance as an alternative technology for biological nitrogen removal from wastewater due to its lower energy, oxygen and carbon requirements [69]. The Anammox process consist of ammonia oxidation in the absence of oxygen but in the presence of nitrite (Equation 1) [70,71]. Therefore, a pre-requisite of the Anammox process is a partial nitrification unit where about 50% of the ammonium is converted to nitrite concentration appropriate to the stoichiometry for the Anammox reaction as shown in Equation 1 [72]. Figure 3 shows the general scheme of Anammox process. The optimal operational conditions for Anammox have been reported at pH=7-8.5; C/N ration 0.6:1; hydraulic retention time of 1 day and temperature 30-37°C [73]. However, Anammox reactors have already been operated at psychrophilic temperatures [74,75].
The feasibility of the process has been demonstrated in laboratory and full scale wastewater treatment [76] and full-scale experiences in the treatment of digestates by Anammox are now become known [77,78]. Anammox is a promising alternative since it has several advantages compared to conventional wastewater treatment bio-systems for N removal [79]. Anammox present ~60% reduction in the oxygen required for nitrification, elimination of organic carbon requirement for denitrification, reduction in the production of biosolids, higher rates of N removal and good coupling with biogas production [80]. The main challenges for implementing Anammox are: (i) the low growth rate of Anammox organisms, causing long start-up periods and the need to ensure bacterial cells retention inside the reactor [81]; (ii) high concentrations of N inside Anammox reactor should be a risk of biomass inhibition, therefore, digestate may be diluted before being supplied to the Anammox reactor, increasing both operational and capital costs; and (iii) digestate sulphide and phosphorus content may interfere in the Anammox process [79].
Absorption
Inorganic nitrogen existing in digestates is an equilibrium between free ammonia (NH3) and ammonium (NH4+). Ammonia recovery from digestates has been investigated with methods such as coupled gas stripping and liquid/solid absorption [82]. Ammonia stripping is carried out by displacing the equilibrium to free ammonia by increase of digestate pH and/or temperature. Ammonia stripping has been trialled using a range of approaches, including with and without solid/liquid separation and using air, nitrogen, steam or biogas as the stripping agent [83]. The ammonia absorption can be done by ion exchange materials or acid solution, of which acid absorption is the widely used since high purity ammonium sulphate crystals can be produced when sulphuric acid solutions are used. Ammonium sulphate crystals isolated from the absorption process is a useful fertilizer with high commercial value [84,85].
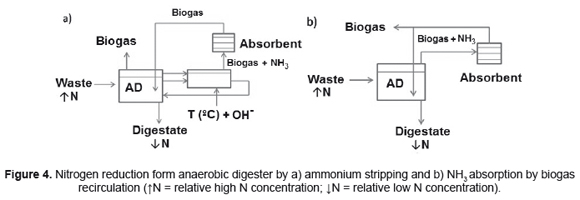
Ammonium plays a vital role as a buffer agent in the AD process [86]. However, high ammonium concentrations are inhibitory for anaerobic biomass, especially aceticlastics methanogens [87]. Consequently, several research efforts have been made to reduce the ammonium concentration in the digester medium [24,64,87]. Chemical absorptions use new reactors configuration to capture the ammonium in a solid or liquid medium. Serna-Maza et al. [88] proposed a side-stream ammonium stripping using thermal alkaline treatment (Figure 4a). In this method, reactor content and biogas are treated in the stripping column and the biogas leaving the stripping column is passed through acid (0.25N H2SO4) traps to remove ammonia, while the biogas and reactor content return to the reactor. Sun et al. [89] reduced ammonia during thermophilic anaerobic digestion of protein rich stillage at a higher organic loading rate (7gL-1d-1) by biogas recirculation on water-washed biogas system (Figure 4b). Wirthensohn et al. [90] tested acidic gel cation exchange resin column (to remove ammonium by ion exchange) after filtration and reverse osmosis at a full-scale AD plant. Resin shows 99% of ammonium removal (27.6g NH4+-N L-1 resin) and present also a regeneration with 3 bed volumes of 2M HCl, recovering 91.7% of the original cation exchange capacity [90].
Phycoremediation
Microalgae biomass has been presented an intense interest application in environmental biotechnology since it can be used for bioremediation of wastewaters [91]. Hence, combining microalgae biomass production with organic wastewater treatment can mitigate: (i) water consume; (ii) substitute for fertilizer requirements of algal cultures by wastewater rich in nitrogen and phosphorus and (iii) algal biomass produced may be further valorized in the bio-fuel production [92- 94]. Nitrogen and phosphorus are considered as essential nutrients for algal cultivation. Nitrogen is directly associated with the primary metabolism of algae as it is the main constituent of nucleic acid and proteins [95]. Phosphorus needs to be supplied as phosphates because phosphorus in other forms may combine with metal ions and get precipitated, thus becoming unavailable to the microalgae [95]. Association of microalgae culture and AD seems a promising technology for sustainable algal biomass and biogas production. The use of digestate liquid fraction for sustaining the growth of microalgae reduces the cost and the environmental impacts associated with the algal nutrient requirements [96]. However, the growth of the microalgal resulting biomass will be driven by light, carbon source, nutrients loads (N and P), trace amount of micronutrients such as metals and vitamins [97]. In the specific use of digestates to microalgae production, the high concentration of ammonium, as well as turbidity, salinity, toxins, etc; present harmful effects on microalgae growth and therefore digestates has to be diluted before used for microalgal cultivation, being the dilution a key element on the economic and operation feasibility [92,93]. Table 2 shows different nutrient removal experiences using microalgae from digestate liquid fraction. In addition to nitrogen and phosphorous, algae also require trace amount of micro-nutrients such as metals (Na, Mg, Ca, Mn, Zn, Cu, Fe and Mo) and vitamins for effective growth [98]. Algae can also be used as bio-sorbent to remove heavy metal ions (e.g. Cu, Pb, Cr, and Sr) [99]. Bio-sortion applied on digestates using algae has several advantages over conventional methods (e.g. chemical reduction, ion exchange, precipitation and membrane separation): (i) low operating cost; (ii) high efficiency in detoxifying heavy metals in low concentration streams; and (iii) no nutrient requirements [100].
Algae growth also becomes attractive for AD systems by the capacity of remove CO2 from biogas. AD produces biogas at CH4 and CO2 concentration between 50-80% and 50-20%, respectively [101]. There are several methods available for the removal of CO2 from biogas (i.e. liquid and solid absorption, pressure swing adsorption, membrane filtration and cryogenic separation) [10]. These processes require considerable amount of energy and their operation may be complex [102]. Microalgae biomass production and biogas upgrading can be also integrated with digestate liquid fraction treatment [103]. Figure 5 shows the microalgae biomass production and biogas upgrading integration.
Membrane separation
Membrane technology has acquired significant relevance in many industry sectors. Membranes can be designed to allow gas transfer between two liquid phases. To accomplish this mass transfer, a microporous hydrophobic membrane separates the two liquid phases, which are an NH3 rich feed and an acidic absorption solution (Figure 6a). The gas filled pores of the membrane are the transfer area. The difference in the NH3 partial pressure between the two liquid phases is the driving force for the mass transfer. Hollow fiber membrane contactors have been used to remove NH3 from anaerobic digestate [109] and also directly from an operating AD reactor [110].
Other uses of membranes in nutrient recovery of anaerobic digestion system are bio-electrochemical systems (BES). The representative system includes microbial fuel cells (MFCs) (Figure 6b), microbial electrolysis cells (MECs) (figure 6c) and microbial desalination cells (MDCs) [111]. The foundation for realizing ammonia recovery in a BES is the fact that ammonium ions can move across ion exchange membrane via either current-driven migration or diffusion [111]. It was found that an MFC could achieve 100% ammonia recovery in efficiency and reduce ammonia inhibition on anaerobic digestion [111,112]. The theoretical analysis of energy consumption and production suggested that ammonia recovery in an MFC had significant energy advantage (with a positive energy balance) [111,113]. One of the key factors in ammonia recovery is the high pH of cathode, which can drive ammonium to ammonia gas [114]
Struvite
Struvite precipitation has been attracting an increased interest as a technique to recover N and/or P, since struvite has a high nutrient value per unit weight (low transport cost) and is highly effective as a slow-release fertilizer [115,116].
Struvite is a crystalline solid phase consisting of magnesium, ammonium and phosphorus in equal molar concentrations and its precipitation naturally occurs, as for Equation 2, when the combined concentration exceeds the solubility product [117,118].
The struvite crystal development occurs in two chemical phases: nucleation (crystal birth) and crystal growth [115]. Several physicochemical parameters influence these mechanisms such as pH [119], super-saturation [120], mixing energy [121], temperature and presence of foreign ions [122]. Taking account of these factors, several struvite crystalliser reactors have been development, mostly continuous flow reactors [115,122-124].
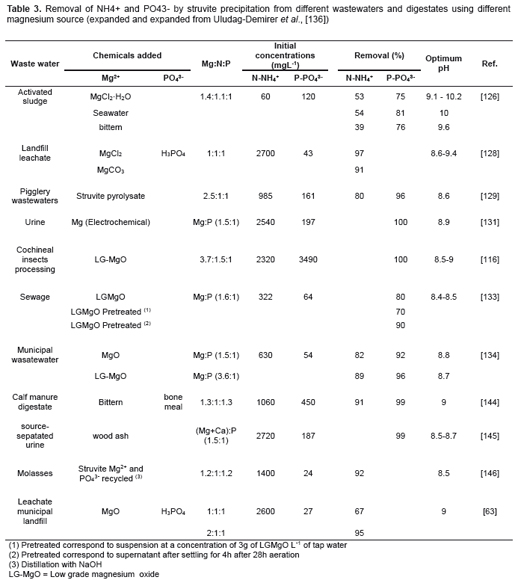
The precipitation of struvite from AD digestates (Figure 7a) normally requires the addition of Mg2+ since their concentration is very low with relation to NH4+ and PO43- concentration [125]. Nevertheless, although the use of struvite for recovering NH4+ and PO43- from wastewaters is technically feasible, it is not widely adopted because of the high costs of chemical compounds [117,126]. Several authors have evaluated the utilisation of alternative magnesium sources such as bittern [127], sea water and brine [128], magnesite [129], magnesite pyrolysate [130], struvite pyrolysate recycling [131] and electrochemical magnesium dosage [132]. Table 3 summarises the removal of NH4+ and PO43- by struvite precipitation from different wastewater and digestates using different magnesium source. Rich-magnesium by-products from the calcination of magnesite have shown good results in struvite precipitation and some advantages in comparison with other Mg2+ sources [117,133,134]. In this topic, Quintana et al. (2008) observed that the origin and the pre-treatment of the by-products have a considerable influence over the reaction time as well as on the quantity and quality of the struvite obtained.
Coupling anaerobic digestion and struvite precipitation in the same reactor have been presented as an alternative approach to further reduce treatment cost (Figure 7b) and, additionally, mitigate ammonium inhibition on anaerobic digestion systems. This approach have been trialed by some researchers on continuous reactors without any negative impact on AD performance and high nutrient recovery (N <50%; P<90%) [136- 138]. However, continued dosing of Mg2+ can led to inhibitory phenomena by extreme pH or cation toxicity as well as high operation costs associated with Mg2+ reagents purchasing [117].
Romero-Güiza et al. [139] found that the use of stabilizing agent (mainly formed of newberyite (MgPO4·3H2O)) formulated with low-grade magnesium oxide by-product, can reduce the ammonium concentration up to 70% and increase the specific biogas production by 40% with a long-term stability.
Struvite has been successfully used as fertilizer on different crops. In fact, struvite is the preferable fertilizer for crops that needs magnesium, like sugar beet [140]. Other favorable aspects of struvite are its low leaching rate (release nutrients slowly during the plant growing season) and that it does not burn the roots like traditional ammonium-phosphate fertilizer [115]. However, in some cases struvite obtained from anaerobic digestates may contain some heavy metals [116], which are incorporated into the struvite crystalline network not only by nucleation, but also during the crystal growth process [129,141- 143]. However, it is unlikely that the heavy metals will exceed limit concentrations for land application [144].
Enhanced phosphorous recovery
Phosphorus is typically present in wastewaters, industrial streams, and anaerobic digestates at low concentrations (10-100mgPL-1). Recovery of phosphates through precipitation with aluminium, iron, calcium and magnesium is technically possible; however aluminium and iron sources are expensive and makes phosphorus unavailable to plants [148]. Calcium phosphates are a poorer fertilizer (particularly in alkaline soils) [149,150], while recovery as struvite requires a higher P concentration in the solution [151]. To solve this problem enhanced biological phosphorus removal (EBPR) is suggested as technology to concentrate phosphorus in order to make phosphorus recovery and reuse feasible. EBPR sludge contains 5-7% phosphorus in contrast with normal activated sludge, ranging 1 and 2% (dry weight) [152]. EBPR relies on polyphosphates accumulating organisms (PAOs) to take up phosphorus from wastewater streams and thus concentrating P in the biomass previous anaerobic digestion [153,154], which digestate can be either directly applied to land or treated to recover P as struvite [155]. Recent advances on EBPR suggest that the main factors involving the technology are: (i) biochemical transformations performed by PAOs, (ii) process design and operation, and (iii) phosphorus recovery from EBRP sludge [152,156]. The EBPR system requires alternating anaerobic and aerobic conditions. In continuous systems this is achieved by spatially dividing the bioreactor into anaerobic and aerobic zones [157-160], while sequencing batch reactors provide anaerobic and aerobic periods in a single vessel [161,162].
Using anaerobic digestates as organic fertilizer or soil conditioner seem to be the best option for its nutrient recycling. However, most digestates are not suitable to be directly applied on land as their properties might cause environmental problems such as ammonia emissions, nitrate leaching and nutrient overdoses. Separation of the solid and liquid fraction is, due to its simplicity, the most widespread digestate treatment method. However, adequate treatments are still required for their correct management. The solid fraction is usually easier to treat, while the liquid fraction requires severe and expensive processes.
Nutrient recovery on anaerobic digestion systems is mainly focus on nitrogen. Nitrogen recovery has been achieved by striping, membrane, electrochemical systems and precipitation technologies. Removing nitrogen from the digester medium rather than from the effluent is an interesting approach since it also allows reducing biomass ammonia inhibition. However, phosphorous recovery has been identified as key a feature in full-scale treatment plants due to phosphorous scarcity and commercial value. In this matter, most research efforts have been made in concentrating phosphorous in polyphosphates accumulating organism's previous anaerobic digestion and subsequent recovery by precipitation. Struvite precipitation is a useful process for both N and P recovery, where research is done to investigate the feasibility of using magnesium by-products as cost-effective Mg2+ source. Finally, the integration of anaerobic supernatant treatment and algae cultivation has been identified as a potential ways to reduce the risk of nitrogen and phosphorus pollution from anaerobic digestion and as a biogas upgrading technology; however further research is required to overcome limiting factors.
Main limitation identified on nutrient recovery from anaerobic digestion systems is the electric and/or chemical requirements, which result in economic unviable processes. Moreover, some of the processes presented are still under development. Another limitation on using anaerobic digestates as organic fertilizer is their marketing. The negative perception by consumers and the competition with conventional fertilizers raise the need to increase digestates marketability and governmental incentives.
[1] Lettinga G. My anaerobic sustainability story. First edit. Netherlands: LeAf; 2014. [ Links ]
[2] Cecchi F, Traverso PG, Mata-Alvarez J, Clancy J, Zaror C. State of the art of R&D in the anaerobic digestion process of municipal solid waste in Europe. Biomass. 1988;16:257- 84. [ Links ]
[3] Mata-Alvarez J, Dosta J, Romero-Güiza MS, Fonoll X, Peces M, Astals S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sustain Energy Rev. 2014;36:412-27. [ Links ]
[4] Vandevivere P, De Baere L, Verstraete W. Types of anaerobic digester for solid wastes. In: Biomethanization of the organic fraction of municipal solid wastes. Mata-Alvarez J, editor. Gran Bretaña: IWA Publishing; 2003. p. 111- 40. [ Links ]
[5] Peces M, Astals S, Mata-Alvarez J. Response of a sewage sludge mesophilic anaerobic digester to short and long-term thermophilic temperature fluctuations. Chem Eng J. 2013;233:109-16. [ Links ]
[6] De Baere L, Mattheeuws B. State of the art of anaerobic digestion in Europe. Int. Water Assoc. 12th World Congr. Anaerob. Dig. 31 Oct - 4 Nov. 2010, Guadalajara , Mex., vol. 32, 2010, p. 1-7. [ Links ]
[7] Flotats X, Bonmatí A, Fernández B, Magrí A. Manure treatment technologies: On-farm versus centralized strategies. NE Spain as case study. Bioresour. Technol. 2009;100:5519-26. [ Links ]
[8] Hengeveld EJ, van Gemert WJT, Bekkering J, Broekhuis a. a. When does decentralized production of biogas and centralized upgrading and injection into the natural gas grid make sense? Biomass Bioenergy. 2014;67:363-71. [ Links ]
[9] Edwards J, Othman M, Burn S. A review of policy drivers and barriers for the use of anaerobic digestion in Europe, the United States and Australia. Renew Sustain Energy Rev. 2015;52:815-28. [ Links ]
[10] Del Valle-Zermeño R, Romero-Güiza MS, Chimenos JM, Formosa J, Mata-Alvarez J, Astals S. Biogas upgrading using MSWI bottom ash: An integrated municipal solid waste management. Renew Energy. 2015;80:184-9. [ Links ]
[11] Astals S, Romero-Güiza MS, Mata-Alvarez J. Municipal solid waste - Energy recovery from the organic fraction based on anaerobic digestion. In: Ferraira G, editor. Altern. Energies, vol. 34. 1st ed. Zaragoza: Springer; 2013, p. 1-26. [ Links ]
[12] Mehta CM, Khunjar WO, Nguyen V, Tait S, Batstone DJ. Technologies to Recover Nutrients from Waste Streams: A Critical Review. Crit Rev Environ Sci Technol. 2014:00-00. [ Links ]
[13] Alburquerque JA, de la Fuente C, Bernal MP. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric Ecosyst Environ. 2012;160:15-22. [ Links ]
[14] Holm-Nielsen JB, Al Seadi T, Oleskowicz- Popiel P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009;100:5478-84. [ Links ]
[15] Batstone DJ, Virdis B. The role of anaerobic digestion in the emerging energy economy. Curr Opin Biotechnol. 2014;27:142-9. [ Links ]
[16] Moscatelli G. Animal manure: Biogas and treatment in Italy. Fira Agrar. St. Miquel, 2015. [ Links ]
[17] Gestore dei Servizi Energitici. Rapporto Statistico - Energia da fonti rinnovabili. Roma, Italy: 2015. [ Links ]
[18] Astals S, Nolla-Ardèvol V, Mata-Alvarez J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: biogas and digestate. Bioresour Technol 2012;110:63-70. [ Links ]
[19] Rodionov A, Nii-Annang S, Bens O, Trimborn M, Schillem S, Schneider BU, et al. Impacts of Soil Additives on Crop Yield and C-Sequestration in Post Mine Substrates of Lusatia, Germany. Pedosphere. 2012;22:343- 50. [ Links ]
[20] Al Seadi T, Lukehurst C. Quality management of digestate from biogas plants used as fertiliser. IEA Bioenergy 2012:4-36. [ Links ]
[21] Demirel B, Göl NP, Onay TT. Evaluation of heavy metal content in digestate from batch anaerobic co-digestion of sunflower hulls and poultry manure. J Mater Cycles Waste Manag. 2012;15:242-6. [ Links ]
[22] Krogstad T, Sogn TA, Asdal Å, Sæbø A. Influence of chemically and biologically stabilized sewage sludge on plant-available phosphorous in soil. Ecol Eng. 2005;25:51-60. [ Links ]
[23] Carlsson M, Lagerkvist A, Morgan-Sagastume F. The effects of substrate pre-treatment on anaerobic digestion systems: a review. Waste Manag. 2012;32:1634-50. [ Links ]
[24] Salminen E, Rintala J. Anaerobic digestion of organic solid poultry slaughterhouse waste - a review. Bioresour. Technol. 2002;83:13-26. [ Links ]
[25] Mata-Alvarez J, Dosta J, Macé S, Astals S. Codigestion of solid wastes: a review of its uses and perspectives including modeling. Crit Rev Biotechnol. 2011;31:99-111. [ Links ]
[26] Alburquerque JA, de la Fuente C, Ferrer- Costa A, Carrasco L, Cegarra J, Abad M, et al. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy. 2012;40:181-9. [ Links ]
[27] Restrepo AP, Medina E, Pérez-Espinosa A, Agulló E, Bustamante MA, Mininni C, et al. Substitution of Peat in Horticultural Seedlings: Suitability of Digestate-Derived Compost from Cattle Manure and Maize Silage Codigestion. Commun Soil Sci Plant Anal 2013;44:668-77. [ Links ]
[28] Abdullahi YA, Akunna JC, White NA, Hallett PD, Wheatley R. Investigating the effects of anaerobic and aerobic post-treatment on quality and stability of organic fraction of municipal solid waste as soil amendment. Bioresour. Technol. 2008;99:8631-6. [ Links ]
[29] Abubaker J, Cederlund H, Arthurson V, Pell M. Bacterial community structure and microbial activity in different soils amended with biogas residues and cattle slurry. Appl Soil Ecol. 2013;72:171-80. [ Links ]
[30] Zirkler D, Peters A, Kaupenjohann M. Elemental composition of biogas residues: Variability and alteration during anaerobic digestion. Biomass Bioenergy. 2014;67:89-98. [ Links ]
[31] Walsh JJ, Jones DL, Edwards-Jones G, Williams a. P. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J Plant Nutr Soil Sci. 2012;175:840-5. [ Links ]
[32] Kirchmann H, Witter E. Composition of fresh, aerobic and anaerobic farm animal dungs. Bioresour. Technol. 1992;40:137-42. [ Links ]
[33] Möller K, Stinner W, Deuker A, Leithold G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr Cycl Agroecosystems. 2008;82:209-32. [ Links ]
[34] Selling R, Hakansson T, Björnsson L. Two-stage anaerobic digestion anables heavy metal removal. Water Sci Technol. 2008;57:553-8. [ Links ]
[35] Alburquerque JA, de la Fuente C, Campoy M, Carrasco L, Nájera I, Baixauli C, et al. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur J Agron. 2012;43:119-28. [ Links ]
[36] Tambone F, Genevini P, D'Imporzano G, Adani F. Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of MSW. Bioresour. Technol. 2009;100:3140-2. [ Links ]
[37] Teglia C, Tremier A, Martel JL. Characterization of solid digestates: Part 2, assessment of the quality and suitability for composting of six digested products. Waste and Biomass Valorization. 2011;2:113-26. [ Links ]
[38] Vintiloiu A, Lemmer A, Oechsner H, Jungbluth T. Mineral substances and macronutrients in the anaerobic conversion of biomass: An impact evaluation. Eng Life Sci. 2012;12:287-94. [ Links ]
[39] Sheets JP, Ge X, Park SY, Li Y. Effect of outdoor conditions on Nannochloropsis salina cultivation in artificial seawater using nutrients from anaerobic digestion effluent. Bioresour. Technol. 2014;152:154-61. [ Links ]
[40] Delzeit R, Kellner U. The impact of plant size and location on profitability of biogas plants in Germany under consideration of processing digestates. Biomass Bioenergy. 2013;52:43- 53. [ Links ]
[41] Tampio E, Ervasti S, Rintala J. Characteristics and agronomic usability of digestates from laboratory digesters treating food waste and autoclaved food waste. J Clean Prod. 2015;94:86-92. [ Links ]
[42] Rigby H, Smith SR. Nitrogen availability and indirect measurements of greenhouse gas emissions from aerobic and anaerobic biowaste digestates applied to agricultural soils. Waste Manag. 2013;33:2641-52. [ Links ]
[43] Vázquez-Rowe I, Golkowska K, Lebuf V, Vaneeckhaute C, Michels E, Meers E, et al. Environmental assessment of digestate treatment technologies using LCA methodology. Waste Manag. 2015;43:442-59. [ Links ]
[44] Gerardo ML, Aljohani NHMM, Oatley-Radcliffe DL, Lovitt RW. Moving towards sustainable resources: Recovery and fractionation of nutrients from dairy manure digestate using membranes. Water Res. 2015;80:80-9. [ Links ]
[45] Wang T, Shao L, Li T, Lü F, He P. Digestion and dewatering characteristics of waste activated sludge treated by an anaerobic biofilm system. Bioresour. Technol. 2014;153:131-6. [ Links ]
[46] Mudragada R, Kundral S, Coro E, Moncholi ME, Laha S, Tansel B. Phosphorous removal during sludge dewatering to prevent struvite formation in sludge digesters by full scale evaluation. J Water Process Eng. 2014;2:37- 42. [ Links ]
[47] Tambone F, Terruzzi L, Scaglia B, Adani F. Composting of the solid fraction of digestate derived from pig slurry: Biological processes and compost properties. Waste Manag. 2015;35:55-61. [ Links ]
[48] Romero-Güiza MS, Peces M, Astals S, Benavent J, Valls J, Mata-Alvarez J. Implementation of a prototypal optical sorter as core of the new pre-treatment configuration of a mechanical-biological treatment plant treating OFMSW through anaerobic digestion. Appl Energy. 2014;135:63-70. [ Links ]
[49] Estevez MM, Sapci Z, Linjordet R, Schnürer A, Morken J. Semi-continuous anaerobic co-digestion of cow manure and steam-exploded Salix with recirculation of liquid digestate. J Environ Manage. 2014;136:9-15. [ Links ]
[50] Nges IA, Wang B, Cui Z, Liu J. Digestate liquor recycle in minimal nutrients-supplemented anaerobic digestion of wheat straw. Biochem Eng J. 2015;94:106-14. [ Links ]
[51] Gong H, Yan Z, Liang KQ, Jin ZY, Wang KJ. Concentrating process of liquid digestate by disk tube-reverse osmosis system. Desalination. 2013;326:30-6. [ Links ]
[52] Massaccesi L, Sordi A, Micale C, Cucina M, Zadra C, Di Maria F, et al. Chemical characterisation of percolate and digestate during the hybrid solid anaerobic digestion batch process. Process Biochem. 2013;48:1361-7. [ Links ]
[53] Campos E, Almirall M, Mtnez-Almela J, Palatsi J, Flotats X. Feasibility study of the anaerobic digestion of dewatered pig slurry by means of polyacrylamide. Bioresour. Technol. 2008;99:387-95. [ Links ]
[54] Meixner K, Fuchs W, Valkova T, Svardal K, Loderer C, Neureiter M, et al. Effect of precipitating agents on centrifugation and ultrafiltration performance of thin stillage digestate. Sep Purif Technol. 2015;145:154- 60. [ Links ]
[55] Møller H. Solid-liquid separation of livestock slurry: efficiency and cost. Bioresour. Technol. 2000;74:223-9. [ Links ]
[56] Lü F, Zhou Q, Wu D, Wang T, Shao L, He P. Dewaterability of anaerobic digestate from food waste: Relationship with extracellular polymeric substances. Chem Eng J. 2015;262:932-8. [ Links ]
[57] Burton CH. The potential contribution of separation technologies to the management of livestock manure. Livest Sci. 2007;112:208- 16. [ Links ]
[58] Parera J, Goya A, Bonmatí A, Riau V, Burgos L. Separadores sólido-líquido. Mundo Ganad. 2015;265:36-41. [ Links ]
[59] Vanden Nest T, Ruysschaert G, Vandecasteele B, Cougnon M, Merckx R, Reheul D. P availability and P leaching after reducing the mineral P fertilization and the use of digestate products as new organic fertilizers in a 4-year field trial with high P status. Agric Ecosyst Environ. 2015;202:56-67. [ Links ]
[60] Saveyn H, Eder P. End-of-waste criteria for biodegradable waste subjected to biological treatment (compost & digestate): Technical proposals. 2014. [ Links ]
[61] Pulvirenti A, Ronga D, Zaghi M, Tomasselli AR, Mannella L, Pecchioni N. Pelleting is a successful method to eliminate the presence of Clostridium spp. from the digestate of biogas plants. Biomass Bioenergy. 2015;81:479-82. [ Links ]
[62] Santi G, Proietti S, Moscatello S, Stefanoni W, Battistelli A. Anaerobic digestion of corn silage on a commercial scale: Differential utilization of its chemical constituents and characterization of the solid digestate. Biomass Bioenergy. 2015;83:17-22. [ Links ]
[63] Di Iaconi C, Pagano M, Ramadori R, Lopez A. Nitrogen recovery from a stabilized municipal landfill leachate. Bioresour. Technol. 2010;101:1732-6. [ Links ]
[64] Yenigün O, Demirel B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013;48:901-11. [ Links ]
[65] Rajagopal R, Massé DI, Singh G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013;143:632-41. [ Links ]
[66] Hadj B, Astals S, Gali A, Mace S, Mata-Alvarez J. Ammonia influence in anaerobic digestion of OFMSW. Water Sci Technol. 2009;59:1153-8. [ Links ]
[67] Velthof GL, Lesschen JP, Webb J, Pietrzak S, Miatkowski Z, Pinto M, et al. The impact of the Nitrates Directive on nitrogen emissions from agriculture in the EU-27 during 2000-2008. Sci Total Environ. 2014;468-469:1225-33. [ Links ]
[68] Lassaletta L, García-Gómez H, Gimeno BS, Rovira JV. Headwater streams: neglected ecosystems in the EU Water Framework Directive. Implications for nitrogen pollution control. Environ Sci Policy. 2010;13:423-33. [ Links ]
[69] Terada A, Zhou S, Hosomi M. Presence and detection of anaerobic ammonium-oxidizing (anammox) bacteria and appraisal of anammox process for high-strength nitrogenous wastewater treatment: A review. Clean Technol Environ Policy. 2011;13:759- 81. [ Links ]
[70] Mulder A, Vandegraaf A, Robertson L, Kuenen J. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol Ecol. 1995;16:177-83. [ Links ]
[71] Van de Graaf A, Mulder A, Debruijn P, Jetten M, Robertson L, Kuenen J. Anaerobic oxidation of ammonium is a biological mediated process. Appl Env Microbiol. 1995;61:1246-51. [ Links ]
[72] Galí A, Dosta J, van Loosdrecht MCM, Mata- Alvarez J. Two ways to achieve an anammox influent from real reject water treatment at lab-scale: Partial SBR nitrification and SHARON process. Process Biochem. 2007;42:715-20. [ Links ]
[73] Sri Shalini S, Joseph K. Nitrogen management in landfill leachate: Application of SHARON, ANAMMOX and combined SHARON-ANAMMOX process. Waste Manag. 2012;32:2385-400. [ Links ]
[74] Lotti T, Kleerebezem R, Hu Z, Kartal B, Jetten MSM, van Loosdrecht MCM. Simultaneous partial nitritation and anammox at low temperature with granular sludge. Water Res. 2014;66:111-21. [ Links ]
[75] Jin R-C, Ma C, Yu J-J. Performance of an Anammox UASB reactor at high load and low ambient temperature. Chem Eng J. 2013;232:17-25. [ Links ]
[76] Ali M, Okabe S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere. 2015;141:144-53. [ Links ]
[77] Abma W, Driessen W, Haarhuis R, van Loosdrecht MCM. Upgrading of sewage treatment plant by sustainable and cost-effective seprate treatment of industrial wastewater. Water Sci Technol. 2010;61:1715- 22. [ Links ]
[78] van der Star WRL, Abma WR, Blommers D, Mulder J-W, Tokutomi T, Strous M, et al. Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 2007;41:4149-63. [ Links ]
[79] Magrí A, Béline F, Dabert P. Feasibility and interest of the anammox process as treatment alternative for anaerobic digester supernatants in manure processing - An overview. J Environ Manage. 2013;131:170-84. [ Links ]
[80] Kartal B, Kuenen J, van Loosdrecht MC. Sewage treatment with Anammox. Science (80- ) 2010;328:702-3. [ Links ]
[81] Ali M, Oshiki M, Rathnayake L, Ishii S, Satoh H, Okabe S. Rapid and successful start-up of anammox process by immobilizing the minimal quantity of biomass in PVA-SA gel beads. Water Res. 2015;79:147-57. [ Links ]
[82] Liu L, Pang C, Wu S, Dong R. Optimization and evaluation of an air-recirculated stripping for ammonia removal from the anaerobic digestate of pig manure. Process Saf Environ. Prot 2015;94:350-7. [ Links ]
[83] Serna-Maza A, Heaven S, Banks CJ. Biogas stripping of ammonia from fresh digestate from a food waste digester. Bioresour. Technol. 2015;190:66-75. [ Links ]
[84] Tao W, Ukwuani AT. Coupling thermal stripping and acid absorption for ammonia recovery from dairy manure: Ammonia volatilization kinetics and effects of temperature, pH and dissolved solids content. Chem Eng J. 2015;280:188-96. [ Links ]
[85] Lee HJ, Oh SJ, Moon SH. Recovery of ammonium sulfate from fermentation waste by electrodialysis. Water Res. 2003;37:1091-9. [ Links ]
[86] Fotidis IA, Karakashev D, Angelidaki I. Bioaugmentation with an acetate-oxidising consortium as a tool to tackle ammonia inhibition of anaerobic digestion. Bioresour. Technol. 2013;146:57-62. [ Links ]
[87] Chen Y, Cheng JJ, Creamer KS. Inhibition of anaerobic digestion process: a review. Bioresour. Technol. 2008;99:4044-64. [ Links ]
[88] Serna-Maza A, Heaven S, Banks CJ. Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour. Technol. 2014;152:307-15. [ Links ]
[89] Sun Z-Y, Yamaji S, Cheng Q-S, Yang L, Tang Y-Q, Kida K. Simultaneous decrease in ammonia and hydrogen sulfide inhibition during the thermophilic anaerobic digestion of protein-rich stillage by biogas recirculation and air supply at 60°C. Process Biochem. 2014;49:2214-9. [ Links ]
[90] Wirthensohn T, Waeger F, Jelinek L, Fuchs W. Ammonium removal from anaerobic digester effluent by ion exchange. Water Sci Technol. 2009;60:201-10. [ Links ]
[91] Hu B, Min M, Zhou W, Du Z, Mohr M, Chen P, et al. Enhanced mixotrophic growth of microalga Chlorella sp. on pretreated swine manure for simultaneous biofuel feedstock production and nutrient removal. Bioresour. Technol. 2012;126:71-9. [ Links ]
[92] Subhadra BG. Water management policies for the algal biofuel sector in the Southwestern United States. Appl Energy. 2011;88:3492-8. [ Links ]
[93] Sander K, Murthy S. Life cycle analysis of algae biodiesel. Int J Life Cycle Assess. 2010;15:704-14. [ Links ]
[94] Ji F, Liu Y, Hao R, Li G, Zhou Y, Dong R. Biomass production and nutrients removal by a new microalgae strain Desmodesmus sp. in anaerobic digestion wastewater. Bioresour. Technol. 2014;161:200-7. [ Links ]
[95] Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, et al. Enhanced CO(2) fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol. 2010;28:371-80. [ Links ]
[96] Vasseur C, Bougaran G, Garnier M, Hamelin J, Leboulanger C, Chevanton M Le, et al. Carbon conversion efficiency and population dynamics of a marine algae-bacteria consortium growing on simplified synthetic digestate: First step in a bioprocess coupling algal production and anaerobic digestion. Bioresour. Technol. 2012;119:79-87. [ Links ]
[97] Franchino M, Comino E, Bona F, Riggio VA. Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere. 2013;92:738-44. [ Links ]
[98] Prajapati SK, Kaushik P, Malik A, Vijay VK. Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: possibilities and challenges. Biotechnol Adv. 2013;31:1408-25. [ Links ]
[99] He J, Chen JP. A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014;160:67-78. [ Links ]
[100]Sheng PX, Ting YP, Chen JP. Biosorption of heavy metal ions (Pb, Cu, and Cd) from aqueous solutions by the Marine Alga Sargassum sp. in single- and multiple-metal systems. Ind Eng Chem Res. 2007;46:2438- 44. [ Links ]
[101]Mata-Alvarez J, Mercé S, Llabrés P. Anaerobic digestion of organic solid wastes . An overview of research achievements and perspectives. Bioresour. Technol. 2000;74:3-16. [ Links ]
[102]Meier L, Pérez R, Azócar L, Rivas M, Jeison D. Photosynthetic CO2 uptake by microalgae: An attractive tool for biogas upgrading. Biomass Bioenergy. 2015;73:102-9. [ Links ]
[103]Serejo ML, Posadas E, Boncz MA, Blanco S, García-Encina P, Muñoz R. Influence of Biogas Flow Rate on Biomass Composition During the Optimization of Biogas Upgrading in Microalgal-Bacterial Processes. Environ Sci Technol. 2015:49(5):3228-36. [ Links ]
[104]Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, et al. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010;101:2623-8. [ Links ]
[105]Levine RB, Costanza-Robinson MS, Spatafora GA. Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biomass Bioenergy. 2011;35:40-9. [ Links ]
[106]Park J, Jin H-F, Lim B-R, Park K-Y, Lee K. Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour. Technol. 2010;101:8649-57. [ Links ]
[107]Yan C, Zheng Z. Performance of photoperiod and light intensity on biogas upgrade and biogas effluent nutrient reduction by the microalgae Chlorella sp. Bioresour. Technol. 2013;139:292-9. [ Links ]
[108]Cai T, Ge X, Park SY, Li Y. Comparison of Synechocystis sp. PCC6803 and Nannochloropsis salina for lipid production using artificial seawater and nutrients from anaerobic digestion effluent. Bioresour. Technol. 2013;144:255-60. [ Links ]
[109]Lauterböck B, Ortner M, Haider R, Fuchs W. Counteracting ammonia inhibition in anaerobic digestion by removal with a hollow fiber membrane contactor. Water Res. 2012;46:4861-9. [ Links ]
[110]Lauterböck B, Moder K, Germ T, Fuchs W. Impact of characteristic membrane parameters on the transfer rate of ammonia in membrane contactor application. Sep Purif Technol. 2013;116:327-34. [ Links ]
[111]Kelly PT, He Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014;153:351-60. [ Links ]
[112]Desloover J, Abate Woldeyohannis A, Verstraete W, Boon N, Rabaey K. Electrochemical resource recovery from digestate to prevent ammonia toxicity during anaerobic digestion. Environ Sci Technol. 2012;46:12209-16. [ Links ]
[113]Haddadi S, Elbeshbishy E, Lee H-S. Implication of diffusion and significance of anodic pH in nitrogen-recovering microbial electrochemical cells. Bioresour. Technol. 2013;142:562-9. [ Links ]
[114]Wu X, Modin O. Ammonium recovery from reject water combined with hydrogen production in a bioelectrochemical reactor. Bioresour. Technol. 2013;146:530-6. [ Links ]
[115]Mukhlesur Rahman M, Salleh MAM, Rashid U, Ahsan A, Hossain MM, Ra CS. Production of slow release crystal fertilizer from wastewaters through struvite crystallization- A review. Arab J Chem. 2014:7(1):139-55. [ Links ]
[116]Uysal A, Yilmazel YD, Demirer GN. The determination of fertilizer quality of th formed struvite from effluent of a sewage sludge anaerobic digester. J Hazard Mater. 2010;181:248-54. [ Links ]
[117]Chimenos JM, Fernández AI, Villalba G, Segarra M, Urruticoechea A, Artaza B, et al. Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-Containing by-product. Water Res. 2003;37:1601-7. [ Links ]
[118]Marti N, Bouzas A, Seco A, Ferrer J. Struvite precipitation assessment in anaerobic digestion processes. Chem Eng J. 2008;141:67-74. [ Links ]
[119]Bouropoulos NC, Koutsoukos PG. Spontaneous precipitation of struvite from aqueous solutions. J Cryst Growth. 2000;213:381-8. [ Links ]
[120]Doyle JD, Parsons SA, Struvite formation, control and recovery. Water Res. 2002;36:3925-40. [ Links ]
[121]Ohlinger KN, Young TM, Schroeder ED. Predicting struvite formation in digestion. Water Res. 1998;32:3607-14. [ Links ]
[122]Le Corre KS, Valsami-Jones E, Hobbs P, Jefferson B, Parsons SA. Struvite crystallisation and recovery using a stainless steel structure as a seed material. Water Res. 2007;41:2449- 56. [ Links ]
[123]Suzuki K, Tanaka Y, Kuroda K, Hanajima D, Fukumoto Y, Yasuda T, et al. Removal and recovery of phosphorous from swine wastewater by demonstration crystallization reactor and struvite accumulation device. Bioresour. Technol. 2007;98:1573-8. [ Links ]
[124]Ali MI, Schneider PA. An approach of estimating struvite growth kinetic incorporating thermodynamic and solution chemistry, kinetic and process description. Chem Eng Sci. 2008;63:3514-25. [ Links ]
[125]Uludag-Demirer S, Demirer GN, Chen S. Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem. 2005;40:3667-74. [ Links ]
[126]Giesen A. Crystallisation Process Enables Environmental Friendly Phosphate Removal at Low Costs. Environ Technol. 1999;20:769-75. [ Links ]
[127]Lee SI, Weon SY, Lee CW, Koopman B. Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere. 2003;51:265-71. [ Links ]
[128]Liu B, Giannis A, Zhang J, Chang VW-C, Wang J-Y. Characterization of induced struvite formation from source-separated urine using seawater and brine as magnesium sources. Chemosphere. 2013;93:2738-47. [ Links ]
[129]Gunay A, Karadag D, Tosun I, Ozturk M. Use of magnesit as a magnesium source for ammonium removal from leachate. J Hazard Mater. 2008;156:619-23. [ Links ]
[130]Huang H, Xu C, Zhang W. Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology. Bioresour. Technol. 2011;102:2523-8. [ Links ]
[131]Yu R, Geng J, Ren H, Wang Y, Xu K. Combination of struvite pyrolysate recycling with mixed-base technology for removing ammonium from fertilizer wastewater. Bioresour. Technol. 2012;124:292-8. [ Links ]
[132]Hug A, Udert KM. Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 2013;47:289-99. [ Links ]
[133]Quintana M, Colmenarejo MF, Barrera J, García G, García E, Bustos A. Use of a byproduct of magnesium oxide production to precipitate phosphorus and nitrogen as struvite from wastewater treatment liquors. J Agric Food Chem. 2004;52:294-9. [ Links ]
[134]Quintana M, Sánchez E, Colmenarejo MF, Barrera J, García G, Borja R. Kinetics of phosphorus removal and struvite formation by the utilization of by-product of magnesium oxide production. Chem Eng J. 2005;111:45-52. [ Links ]
[135]Quintana M, Colmenarejo MF, Barrera J, Sánchez E, García G, Travieso L, et al. Removal of phosphorus through struvite precipitation using a by-product of magnesium oxide production (BMP): Effect of the mode of BMP preparation. Chem Eng J. 2008;136:204-9. [ Links ]
[136]Lee J, Choi C, Lee M, Cheng I, Kim D. A study of NH3-N and P fixation by struvite formation in hybrid anaerobic reactor. Water Sci Technol. 2004;49:207-14. [ Links ]
[137]Uludag-Demirer S, Demirer GN, Frear C, Chen S. Anaerobic digestion of dairy manure with enhanced ammonia removal. J Environ Manage. 2008;86:193-200. [ Links ]
[138]Yilmazel YD, Demirer GN. Removal and recovery of nutrients as struvite from anaerobic digestion residues of poultry manure. Environ Technol. 2011;32:783-94. [ Links ]
[139]Romero-Güiza MS, Astals S, Chimenos JM, Martínez M, Mata-Alvarez J. Improving anaerobic digestion of pig manure by adding in the same reactor a stabilizing agent formulated with low-grade magnesium oxide. Biomass and Bioenergy. 2014;67:243-51. [ Links ]
[140]Gaterell MR, Gay R, Wilson R, Gochin RJ, Lester JN. An Economic and Environmental Evaluation of the Opportunities for Substituting Phosphorus Recovered from Wastewater Treatment Works in Existing UK Fertiliser Markets. Environ Technol. 2000;21:1067-84. [ Links ]
[141]Münch EV, Barr K. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams. Water Res 2001;35:151-9. [ Links ]
[142]Liu Y, Kwag J-H, Kim J-H, Ra C. Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination. 2011;277:364-9. [ Links ]
[143]Song Y, Qiu G, Yuan P, Cui X, Peng J-F, Zeng P, et al. Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions. J Hazard Mater. 2011;190:140-9. [ Links ]
[144]Marcato CE, Pinelli E, Pouech P, Winterton P, Guiresse M. Particle size and metal distributions in anaerobically digested pig slurry. Bioresour. Technol. 2008;99:2340-8. [ Links ]
[145]Siciliano A, Rosa SD. Recovery of ammonia in digestates of calf manure through a struvite precipitation process using unconventional reagents. Environ Technol. 2014;35:841-50. [ Links ]
[146]Sakthivel SR, Tilley E, Udert KM. Wood ash as a magnesium source for phosphorus recovery from source-separated urine. Sci Total Environ. 2012;419:68-75. [ Links ]
[147]Türker M, Celen I. Removal of ammonia as struvite from anaerobic digester effluents and recycling of magnesium and phosphate. Bioresour. Technol. 2007;98:1529-34. [ Links ]
[148]Pritchard D, Penney N, McLaughlin M, Rigby H, Schwarz K. Land application of sewage sludge (biosolids) in Australia: risks to the environment and food crops. Water Sci Technol. 2010;62:48-57. [ Links ]
[149]Erdincler A, Seyhan L. Agricultural use of municipal wastewater sludges: phosphorus availability of biological excess phophorus removal sludges. Water Sci Technol. 2006;54:131-8. [ Links ]
[150]Bauer P, Szogi A, Vanotti M. Agronomic effectiveness of calcium phophate recovered from liquid swine manure. Agron J. 2007;99:1352-6. [ Links ]
[151]Pastor L, Marti N, Bouzas A, Seco A. Sewage sludge management for phosphorus recovery as struvite in EBPR wastewater treatment plants. Bioresour. Technol. 2008;99:4817-24. [ Links ]
[152]Yuan Z, Pratt S, Batstone DJ. Phosphorus recovery from wastewater through microbial processes. Curr Opin Biotechnol. 2012;23:878-83. [ Links ]
[153]Lv X-M, Shao M-F, Li C-L, Li J, Gao X, Sun F-Y. A Comparative Study of the Bacterial Community in Denitrifying and Traditional Enhanced Biological Phosphorus Removal Processes. Microbes Environ. 2014;00:261-8. [ Links ]
[154]Huang W, Huang W, Li H, Lei Z, Zhang Z, Tay JH, et al. Species and distribution of inorganic and organic phosphorus in enhanced phosphorus removal aerobic granular sludge. Bioresour. Technol. 2015;193:549-52. [ Links ]
[155]Oehmen A, Lemos PC, Carvalho G, Yuan Z, Keller J, Blackall LL, et al. Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res. 2007;41:2271-300. [ Links ]
[156]Rashed EM, Massoud M. The effect of effective microorganisms (EM) on EBPR in modified contact stabilization system. HBRC J. 2014:1-9. [ Links ]
[157]Xu X, Liu G, Zhu L. Enhanced denitrifying phosphorous removal in a novel anaerobic/ aerobic/anoxic (AOA) process with the diversion of internal carbon source. Bioresour. Technol. 2011;102:10340-5. [ Links ]
[158]Sibag M, Kim HS. Nitrification denitrification enhanced biological phosphorous removal (NDEBPR) occurs in a lab-scale alternating hypoxic/oxic membrane bioreactor. Bioresour. Technol. 2012;104:173-80. [ Links ]
[159]Jafarzadeh Ghehi T, Mortezaeifar S, Gholami M, Rezaei Kalantary R, Mahvi AH. Performance evaluation of enhanced SBR in simultaneous removal of nitrogen and phosphorous. J Environ Heal Sci Eng. 2014;12:1-7. [ Links ]
[160]Yan P, Guo J-S, Wang J, Chen Y-P, Ji F-Y, Dong Y, et al. Enhanced nitrogen and phosphorus removal by an advanced simultaneous sludge reduction, inorganic solids separation, phosphorus recovery, and enhanced nutrient removal wastewater treatment process. Bioresour. Technol. 2015;183:181-7. [ Links ]
[161]Xu D, Chen H, Li X, Yang Q, Zeng T, Luo K, et al. Enhanced biological nutrient removal in sequencing batch reactors operated as static/ oxic/anoxic (SOA) process. Bioresour. Technol. 2013;143:204-11. [ Links ]
[162]Wang Z, Meng Y, Fan T, Du Y, Tang J, Fan S. Phosphorus removal and N2O production in anaerobic/anoxic denitrifying phosphorus removal process: Long-term impact of influent phosphorus concentration. Bioresour. Technol. 2015;179:585-94. [ Links ]