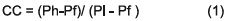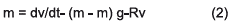Introduction
The term drag in mineral flotation process can be defined as the method to mechanically transfer the suspended particles in the pulp using air bubbles and foams, so that the particles can be dragged in the froth zone of the flotation pulp and thus achieve a concentrate [1-2]. In this process; several factors have influence in the buoyancy and recovery of mineral of interest as: pulp density, gran size, mineral compound, rheology, external conditions, froth structure, and others [3-4]. The particle size of the particle and the froth stability, viscosity and pulp density during the process relevant factors during the process in the copper concentration process by flotation [2-5] Generally, flocculants and peptizes during flotation are applied to allow clays to be included into the pulp in suspended form. This reagent can promote the precipitation of the ore and generate losses during the process. [6] The copper minerals are usually of hydrothermal character comprised of sulphur minerals of Pb, Fe, Zn, Cu and precious minerals such as gold and silver, as well as silica, formed by quartz that could contain sludges and clays in formations of phyllo-silicates in tetrahedral and octahedral silica sheets of alumina in certain proportions with varying sizes, depending on their training [7-8]
Structurally, the layers generated between minerals permanently apply negative charges on their basal surfaces, which can be neutralized with interminable cations provided by alkaline substances that can form ions such as Na+, Ca+ and Mg+ [9-10]. This factor does not compromise only in the neutralization of clay compounds but also in the stabilization of pH inside the pulp. For this, reason alkaline reagents in form of hydroxides and carbonates are applied, handled not only as a neutralizer clay mineral but also in the variation of acidity of the pulp during the troth flotation. The variation of pH in the pulp by this method contributes directly to the flotation of minerals depending of their formation [12] but, in turn, it can be generated the precipitation of valuable mineral. This can lead the addition of flocculants reagents, to the precipitation of clays that carry the ore of interest [9]. The addition of peptizing reagents generates colloidal particles which can be more easily dispersed in the pulp. The clays in colloidal form are suspended inside the water and incorporates the ore of interest avoiding copper recovery during the flotation stage [9]. This effect is reduced by adding saline solution and in this way reducing the swelling ability of the clay in the flotation pulp [10;13]. A mechanism used for the process of minerals dewatering is by gravimetric concentration from Jig type hydraulic screens where, by means of pulsations, the variation and the movement generated within a chamber allows the separation by gravity of the materials present in the sample [14-16], eliminating the clay material of the sample and maintaining the mineral of interest in the lower part of the sample [13;17-18]
In this study, a JIG type hydraulic screen was used as a mechanism for the removal of clays to dewax and reduce the losses of copper ore in the glues, useful at an industrial level [19]. In the JIG gravimetric concentration, the mineral particles move in a pulsating water flow resulting in the end of the process in a stratification of particles of different densities and sizes. The stratification of the particles into the equipment in a complex multiphase flow field. The particles undergo various hydrodynamic forces caused by the movement of the fluid, giving rise to the various paths that depend on the field of liquid velocity and the properties of the particles. Various operational variables affect the movement of particles, including water and mineral feed flow, amplitude and frequency of pulsation of the fluid, among others [20].
Materials and methods
This study was carried out in 3 stages. The first part consisted in the material selection, sampling and mechanical preparation; The second part corresponds to the realization and comparison of the process of dewatering of the mineral in concentrator type JIG and inside a Denver cell followed by froth flotation. The third part is related to the analysis of each one of the samples obtained in the previous stages, required in the process of concentration by foam flotation.
Sampling
In the area, a random sample was taken and 80kg of sample where were taken and then dried in a muffle oven at 110 °C for 6 hours; This mineral contained 9.12 % moisture to make the analyses on a dry basis. The dry ore was quartered in rifle until 5.0Kg left that were used in the investigation, this sample is a copper mineral (chalcopyrite) of hydrothermal formation with presence of pyrite, silica and clays. The sample was taken and selected according to ASTM C702 / C702M-11.
Crushing and grinding
The material was prepared by means of a closed-loop Blake jaw crusher with an aperture of 1/2". A ball mill with a maximum capacity of 1kg of ore was used for the grinding process. The material obtained from the grinding process was sieved between meshes -80 and +230. According to ASTM E-11. The samples obtained, were taken to the process of removal of clays in concentrator JIG and cell Denver.
Gravimetric concentrator type jig
In the gravimetric concentration, a jig piston concentrator was used. The conditions of use were 200 beats per minute, aperture sieve of 1.651mm, double layer of metallic spheres as bedding material, water injection of 0.5Lmin-1, feed of pulp of 0.4Lmin-1 with a pulp of 20 % solids
The gravimetric concentrating equipment separates into two products according to the specific gravity of the minerals. The equipment was designed for mineral processing of 1/8 "or less with a capacity of 20 kgh-1 and a flow rate of 1 to 3 gallons min-1, with an adjustable displacement of 1/2".
The relation between the gravity difference of the minerals related to the specific gravity of the fluid where, when pf = 1, the ratio becomes the free settlement relation of the mineral species. It is necessary to consider as criterion of concentration CC. Likewise, when it is different from 1, the relationship is a hampered settlement [21]:
where,
h = specific gravity of the heavy mineral, kg. m-3 x 10-3
l =specific gravity of the light mineral, kg. m-3 x 10-3
f = fluid density, kg. L-1.
Due to the influence of the specific density and the force exerted by the piston, the particles are carried upwards until eventually their velocity is brought to zero and is formed an initial acceleration whit the descent of the particles in relationship to the gravity and the weight of the particles.
In initial conditions R(v) is despised, whereby.
Where,
m= weight of the particle, kg.
m= Mass of displaced fluid, kg.
v = Velocity of the particle, m/s.
t = time, s
g = gravitational acceleration, m/s2.
R (v) = drag force.
s = specific gravity of the particle, Kg/m3.
f = specific gravity of the fluid. Kg-1
the relationship of grain size in the particles is taken from the relation of free settlement where,
Where n is a coefficient that could variate between 1 and 0.5
n = 1 for Newtonian regime
n = 1/2 for Stokian regime
2.4. Denver concentrator cell.
The Cell managed in the froth flotation test was from a Denver type Flotation cell whit a capacity of 2000 ml; the froth flotation tests were performed from pulps with 20 per cent of solids by weight with distilled water [22].
Sludge remotion process
Are used two types of techniques for the sludge and clays remotion process of chalcopyrite ore (Denver Cell and Hydraulic Screen Jig), where is observed the variation of the particle size of the particle into the mineral in relation to the process managed for the sludge remotion of the samples. Is observed the reduction in value material losses in relation to the specific density of the mineral's particles, as well as, is present a significant improvement during the process of remotion of sludges and clays in the samples worked in screen type Jig than those that were worked inside the Denver flotation cell [4;19].
Chalcopyrite froth flotation
For the froth flotation, a Denver cell was used, where the pH was regulated to 10 through the application of Dissolved Sodium hydroxide. The pulp is made with 20 % of solids in weight with distilled water. As additional reagents, sodium silicate was used to depress the silica silicates into the pulp, and sodium carbonate was used as peptizing agent until the pulp pH of 12 [23]. Xanthate and A-31 as collectors and activators respectively added in relation 2:1 during the froth flotation process. Methyl iso-butyricarbinol (4-methyl-2-pentanol) was used as a low foaming agent. The process was performed at room temperature 17 °C for each of the samples. [12;24-25]
The flotation process in Denver cell counted with the rougher and scavenger stages where the greatest possible recovery of Chalcopyrite ore was sought in the samples having as key parameters the main characteristics of the particles [4]. For the case of the samples managed by the sludge removal process in hydraulic screen Jig did not require an exhaustive stages of second flotation (scavenger) during the concentration process by froth flotation of mineral.
Characterization
The samples obtained during the sampling and the froth flotation process were analyzed through X-ray diffraction XRD Panalytical, worked with: cobalt filament lamp with Wavelength of 1.75 A; Variable angle Pixel detector with Bragg-Brentano configuration and X-ray Fluorescence using a Philips RX-240 sequential spectrometer. The dispositive was suministred by the investigation centre INCITEMA at Universidad Pedagógica y Tecnológica de Colombia.
Results and discussions
Mineral compound
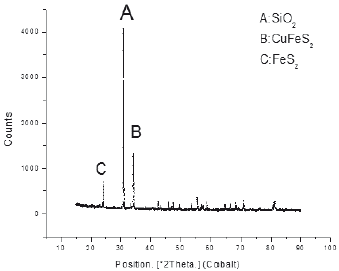
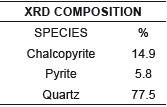
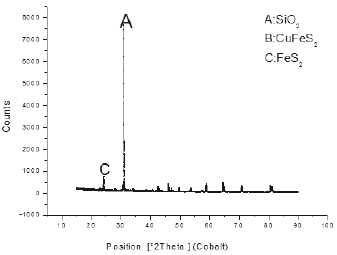
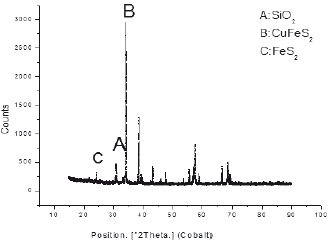
One of the techniques used to identify mineralogical species was X-ray diffraction (XRD); Presenting a high percentage of silica in the sample worked with about 77.5%, chalcopyrite of 14, 9% and 5.8% of pyrite inside the sample. The high presence of silica necessitates the use of concentration (flotation) of the mineral to eliminate it and raise the content of chalcopyrite containing copper in this mineral; In addition, it can be concluded that it is a sulphide mineral, which has considerable presence of clays and carbonaceous material.
To corroborate the previous information, a second mineral characterization method was used using the x-ray fluorescence technique XRF using a Philips RX-240 sequential spectrometer, finding sulfuric mineralogical species in the company of clays (aluminosilicates). The spectrum shows as major compounds iron and silicon in the form of pyrite and silica, followed by copper sulphide in the form of chalcopyrite CuFeS2.
The spectra show that major compounds are iron and silicon in the form of pyrite and silica, follow by copper sulphide in the form of chalcopyrite CuFeS2. When comparing the two techniques of characterization, sulfuric mineralogical species have the same percentage better identifying the clays (aluminosilicates) by XRF; As the primary concentration technique, the use of the JIG gravimetric concentration was proposed to remove aluminosilicates from the mineral.
Jig gravimetric concentration
In this process it was possible to eliminate 99.2% of the total content of the clays in the mineral. To obtain this result, different tests were made varying the conditions of use of the machine and the number of solids in the pulp until obtaining 20% of solids with which the greater elimination of this species is achieved; Gradually increasing the percentages of other mineralogical species that require another type of concentration. The mineralogical phase containing copper (chalcopyrite) is concentrated from 14.9% to 23.01%.
It is noted that the tails obtained in this process the content of chalcopyrite are below 0.110% and the rest is formed by the aluminosilicates in small amounts of pyrite and silica.
Flotation
The conditions handled for the process of concentration by froth flotation were the same in all the tests registered in numeral 2.6. As the pH increases, a smaller number of foaming agents must be added during the process. In addition, the addition of Xanthate and A-31 is decreased during the Rougher and Scavenger processes. On the other hand, the addition of sodium silicate as a depressant must be increased during the second concentration step Scavenger. The concentrate has the following mineralogical composition:
By this method of concentration, it was possible to increase the chalcopyrite by 54.2% and to reduce the silica by 22.8%.
Most of the tails correspond to bargain or sterile material and has been achieved by almost eliminating the chalcopyrite and, therefore, the study metal; Also, the high concentration of silica that at a certain moment can affect the metallurgical process of copper production
Flotation is a method of concentration that applies in a high percentage in the removal of sterile of this mineral and is managed to elevate in a high percentage the copper content, ready for metallurgical processes of obtaining the metal of interest.
Process analysis
Characterization of the mineral: When analyzing the X-ray diffraction (XRD) and X-ray fluorescence (XRF) of the mineral composition of the copper ore, it is found that the great majority of the species of interest for the investigation contains sulphur for this reason it is twelve that is A sulphurous mineral of copper.
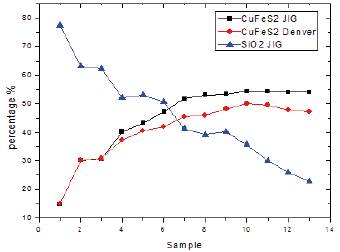
Concentration: The following graph shows the process as a gain of chalcopyrite in concentrates and losses of the same in the tails or tail with respect to the time in the gravimetric concentration and the foam flotation of the sulphide copper ore. Red line inside the graph shows the poor efficiency of the foam concentration in the mineral without having had previous gravitational concentration; The chalcopyrite reaches an average of 47.5% with significant losses in the sludge; The black line shows the importance of the use of concentrator Jig eliminating all the low density clay material that is contained within the mineral to be treated and to be subjected to concentration by foam raises the concentration efficiency of metal or mineralogical study with content Chalcopyrite of 54.1%.
The highest degree of concentration was achieved when working with a particle size of -100 and +120 mesh with a pulp of 20% solids and a working time of 30min for each sample in both types of concentration, achieving a degree of Recovery of chalcopyrite of 98.9%.
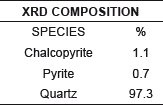
The highest concentration of foam flotation was achieved when the mineral was previously concentrated gravimetrically; With a residual of 23.0% silica in the concentrate; The tails in this process have a major component of 97.30% silica.
Conclusions
A higher degree of sludge removal was observed within the JIG type hydraulic screen than those samples that were treated within the Denver cell.
The sludge and clays removal prior the foam flocculation concentration method helps to increase the throughput of the process and to considerably decrease the number of reagents required for the scavenger stage flotation process.
There was a relevant increase of percentage of concentrates in the samples taken to concentration stage by flotation by foam when they have been previously processed in by clays removal. In the same way, a significant improvement and a reduction in ore losses during the previous process when being handled in Jig type concentrator was observed.
Any material containing clay materials is important to perform a gravimetric concentration process prior to flotation concentration processes.