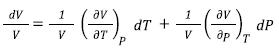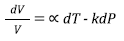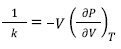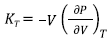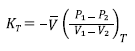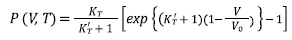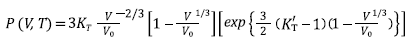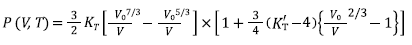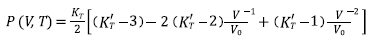Introduction
There is an increasing interest in the development of equations of state for solid, liquid, and vapour phases [1-6], to improve process efficiency and yield. Many authors have studied methods for the distinction of a liquid phase from a vapour pase without reference to its saturation properties. Poling et al. [7], used the following criteria based on the isothermal compressibility (k) to identify the phase of a fluid. The method works only at low pressures and away from the critical point where the isothermal compressibility of the vapour and liquid phases in equilibrium are very different [8].
In the present work, we have studied the mechanical propierties, especially volume compression (V/ V0) of three acids with a long carbon chain by extending the bulk modulus property in liquid phase to the solid phase. Then, it was feasible to widely used Tait, Vinet, Murnaghan and Kholyia Equation of State (EOS) [8-13]. Calculated results were evaluated regarding the conformation of molecule studied but not compared with the experimental values due to they do not exist.
It is observed that, Murnaghan formulation [8-10], gives almost the same results as reported by input parameters, the values of bulk modulus and its first-order pressure derivative for the acids stearic, palmitic and lauric, which were taken from extending results from P-R equation [7], at low and constant temperature. The values of bulk modulus and its first-order pressure derivative for acids with long carbon chain was taken to be in the range of 2.2 <KT<3.3 GPa and 18< KT’<61.
Theory
Usually the study of the influence of pressure (P) under the volume (V) comes from relations of condensed matter and is done by the equation of states. There are many equations of state described in the literatura but there is still a severe need to search a simple and most suitability equation of state which is aplicable in the entire range of compressions. It is observed that most EOS give the same result under small compression.
Franco and coworkers 2017 [14], have been demonstrated that P-R (Peng-Robinson) Equation (7), presented reliable results when used to estimate molar volumes of liquid and solid phase for stearic, palmitic, and lauric acids. Estimates for termal expansivity and isothermal bulk modulus can be done for those systems providing reasonable results.
For different phases, the volume (V) can be written by the Equation (1),
Or substituting the definitions of expansivity and compressibility, the Equation (1) becomes Equation (2),
Where α is the thermal expansion coefficient and k is the compressibility. Considering the second parameter k and inverting it, thus:
Then this inversion is called bulk modulus (K T ) and is defined as [13,14],
Where T is the temperature of the system and the derivative has to be calculated using an EOS.
Equation (4), has been applied to the solid systems such as NaCl and MgO at relatively low pressure, the calculated values of the isothermal bulk modulus was found agree with available experimental data in the pressure range.
Jayanti and Venkatarathnam 2016 [8], have shown the variation of isothermal compressibility (k T ) of solid carbon dioxide with the temperature at specified pressures, along with that of the vapour and liquid phases.
The calculations with the vapour phase are limited to the sublimation region, and those with the liquid phase to the solid melting region. It can be seen from their work that the slopes of the isobars are of the same sign for both the solid and the liquid phases, while those of the vapour are of the opposite sign (relative to the solid and liquid phases). A similar result was also obtained experimentally by Heberlein et al. [15], in the case of solid nitrogen.
In this work bulk modulus parameter K T (T,P) estimated for the líquid phase is extended to solid phase. All the values are obtained using the results from the Peng-Robinson equation of state, with the help of an approximation of Equation (4), for some fatty acids. Then, considering two different states, with p1 and p2, the result was obtained approximately by:
And
Where V1 and V2 are calculated using the P-R equation for each state condition.
There are no experimental data reported by any researcher based on the theoretical or experimental measurement of thermodynamic properties at any pressure. Then, the results will be applied to estimate the parameters used in the appropriate equations for the solid phase.
Equations (7), (8), (9), and (10) are Tait, Vinet, Birch-Murnaghan and Kholiya et al. 2014, equation of states [16-19], respectively, and here K T and K’ T are the bulk modulus and its first-order pressure derivative at specific p and T, respectively:
Results and discussion
Table 1 illustrates the properties of the compounds used in the equation of state in this work. Test of validity of P-R [7], and selection of one to be used was done previously. The values of the liquid molar volume (V, cm3ml-1), the isothermal bulk modulus (KT) and the thermal expansion coefficient α (T, P) (K-1) were determined.
Table 1 Properties for the chemicals [19].
| Chemical | pc/atm | Tc/K | ω | Teb/K | Ttrp/K | Pptr/bar | VS 20°C |
| Lauric acid C12H24O2 | 18.68 | 743.43 | 1.000 | 571.00 | 316.98 | 198.61 | |
| Palmitic acid C16H32O2 | 14.68 | 785.22 | 0.999 | 612.15 | 335.66 | 8.266E-08 | 258.85 |
| Stearic acid C18H36O2 | 13.26 | 805.09 | 1.018 | 634.20 | 342.49 | 4.266E-08 | 282.03 |
The density results obtained from P-R Equation are reported in Franco et al. 2017 [14], along with the input data on experimental density. For the sake of comparison, the calculated results presented by Lauric, Palmitic, and Stearic acids are also listed in their work.
According to Mao et al. 2015 [20], although K S (adiabatic bulk moduli) increases almost linearly with pressure, at 300 K exhibits a downward tren with pressure due to the softening of the shear moduli. Elevating temperature at a given pressure decreases all of the elastic moduli, it is also observed in Table 2. In general, most of the elastic moduli exhibit similar pressure dependence at the investigated temperature range. It is important to mention that Gal 2021 [23], has analysed the phases α, ß, ß› and ß” which showed different bulk moduli K T and K To ' and different zero pressure volumes (Vo) than those reported in the literature.
Table 2 K T (GPa) parameters estimated by using data calculated in this work.
| T (K) | P(GPa) | Stearic acid | Palmitic acid | Lauric acid |
| 0.1 | 2.23 | 2.461 | 2.595 | |
| 350 | 1.0 | 1.00 | 2.319 | 2.657 |
| 2.0 | 1.00 | 2.407 | 2.517 | |
| 0.1 | 1.628 | 1.673 | 1.913 | |
| 400 | 1.0 | 1.644 | 1.699 | 1.900 |
| 2.0 | 1.676 | 1.719 | 1.933 | |
| 0.1 | 1.221 | 1.264 | 1.385 | |
| 450 | 1.0 | 1.238 | 1.267 | 1.389 |
| 2.0 | 1.234 | 1.268 | 1.471 |
The values of KT have been predicted through P-R Equation using Equation (5), by taking all input parameters from Table 1 for three different fatty acids. The results are shown in Table 2 as a function of temperature in three pressures: 0.1, 1.0 and 2.0 GPa. As the carbon chain decreases, the effect of temperature in the bulk modulus is similar for the three acids. In general, the pressure effect is slightly observed. Apart from Stearic acid at 350 K, it is noted that KT remained almost constant in the range of pressure studied. As the temperature increases, the KT value decreases. This was observed in most of the cases studied. For a given temperature, K T decreases as the carbon chain decreases. By comparing the results, in the pressure ranges of Table 2, it can be seen that the K T value remains practically unchanged which results in the derivative K’ T around of: 18.2, 29.9 and 61.0 for each acid in the same sequence presented in Table 2.
Pandey et al, 2015 [21], point out that the input values of K T (GPa) and K’ T for carbon nanotube bundles, individual carbon nanotubes, and graphite are taken from literature and is cited here, in pairs: (37.0;11.0), (230; 4.5) and (33.8, 8.9), respectively. They have presented the calculated values of volume compression with pressure for those systems using the different of states: Suzuki, Shanker, Tait and Murnaghan Equation of State (EOS).
To study the compression behavior the present study requires two input parameters, namely, K T (GPa) and K’ T . In the literature, there are no experimental values for the long carbon chain acids. Then, an extrapolation of data from the liquid phase was done by observing the recommendation in the Jayanti and Venkatarathnam’work. Sigh et al. 2021 [24], determined the MechElastic package that can plot the equation of state (EOS) curves for energy and pressure for a variety of EOS models such as Murnaghan, Birch, Birch- Murnaghan, and Vinet, by reading the inputted energy/pressure versus volume data obtained via numerical calculations or experiments. This work will be done soon and is particularly useful for the high-throughput analysis of elastic and mechanical properties of materials.
Figures 1-3 shown the behavior of V/Vo as a function of the pressure for the three acids used in this work.
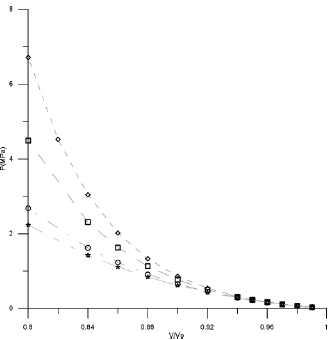
Figure 1 Compression behavior of Stearic acid using different EoSs (◊Tait Equation, □Vinet Equation, ○Birch-Murnagham Equation, ⃰ Kholiya et al. 2014).
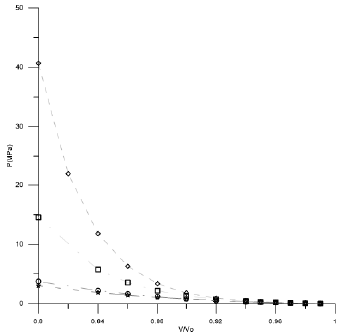
Figure 2 Compression behavior of Palmitic acid using different EoSs. (◊ Tait Equation, □ Vinet Equation, ○ Birch-Murnagham Equation, *Kholiya et al. 2014,).
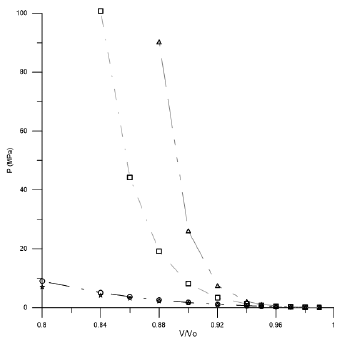
Figure 3 Compression behavior of Lauric acid using different EoSs. (◊Tait Equation, □Vinet Equation, ○ Birch-Murnagham Equation, * Kholiya et al. 2014).
In the low compression range by using the suitable cited of input parameters, all the equations can provide similar theoretical results as at low pressure all EOSs give almost the same results. But as pressure increases the difference of the values calculated from different EOS increase. For high compression the small difference in the carbon chain has a hard effect on the calculated values and in the form of an EOS becomes more significant, different EOSs give different results. From Figures 1 and 2 it is clear that Tait EOS, Vinet EOS, Birch-Murnaghan EOS and Kholiya EOS give results compatible when V/Vo is below 0.92 while Figure 3 for Lauric acid gives low results and hence fails a lot at high pressure. It is pertinent to mention here that the selection of other set of input parameters also provides analogous outcome.
Latimer et al. 2018 [25], have commented that despite over a century of theoretical development and experimental testing of energy-volume (E-V) EOS for solids, there is still a lack of consensus about which equation is indeed optimal, as well as to what metric is most appropriate for making this judgment. Recently, the literature [26], has shown that the characteristic parameters P* V* of the modified Vander Walls EOS depend only on the nature of the material and not on its state (liquid, glassy, solid of different structure), then it would be interesting to verify this new EOS for acids.
Conclusions
Peng-Robinson equation of state was approved to represent the pVT behavior for at least three fatty acids such as Lauric, Palmitic and Stearic. Based on this finding, the bulk modulus parameters and its first derivative was estimated and their results applied to study the behavior of solid compression. Results have shown that similarly to other materials the estimated parameter can be calculated by extrapolation of data from liquid phase but have considerable influences with the carbon long chain. Also, at high pressure, it is really necessary to have experimental data to decide the best EoS to be chosen.
Notation
k-bulk modulus, GPa;
P-pressure, Pa;
T-temperature, K;
V-molar volume, cm3mol-1;
ω-admensional acentric fator;
κ-compressibility, Pa-1.
α-isothermal expansivity, K-1.
Subscripts and superscripts
c-critical;
eb-boiling point;
trp-triple point;
S-solid;
S-adiabatic;
0-at T0 temperature;
T-isothermal;
‘-derivative.