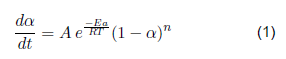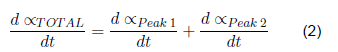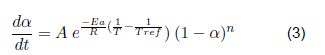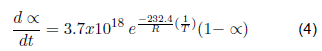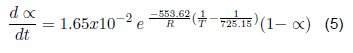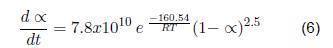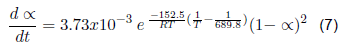Introduction
The advancement of medicine increasingly encompasses a more rigorous field in the management of biosafety measures. Health care can be provided for the prevention, recovery, and rehabilitation of the comprehensive health of the population.
Handling disposable and personal-use material is part of the essential requirements; on the other hand, it involves generating a large amount of waste. These inputs are also used in research centers, the production of biological elements, mortuary centers, and veterinary and nursing homes [1].
In Ecuador, bio-medical materials [2] are considered hazardous waste [3]. In addition to regulations, international standards are described by the World Health Organization (WHO) [4]. These require special handling to avoid the transmission of diseases and contamination of the environment. Therefore, its correct final disposal is of significant importance for national and international organizations, leading to implementing policies and security measures to manage waste from production, storage, collection, transport, treatment, and final disposal. Unlike general waste, infectious waste requires specialized treatments. Therefore, it could cause health risks to living beings and environmental contamination. Based on the information reported by the GADM (Governments Autonomous Municipal Decentralized), it was determined that each inhabitant of Ecuador produces an average of 0.83 kg of solid waste per day in the urban sector. In 2020, 12,612.5 tons per day were collected. Near 85.6 % were collected in an undifferentiated manner, and 14.4 % in a differentiated way. In the final disposal phase, it was reported that 50.5 % of the GADM disposed of urban solid waste in landfills, 31.4 % in pop-up cells, and 18.2 % in dumps [5]. The technologies used in the country for their treatment are inefficient since they do not meet the demand for waste that originates and the disposal of sites destined for sanitary landfills is increasingly scarce [6].
Due to the pandemic, the production of sanitary materials such as face masks has increased significantly in China, where in March 2020 alone, it exported more than 3.86 billion units to various countries [7]. In addition, enterprise 3M [8] has increased its production of N95/FFP2 type face masks since January 2020 to reach 2000 million units per year by 2021 in its various manufacturing facilities to meet product demand. Because biohazardous waste represents a high biohazard, it must be disposed of in appropriate containers. National Risk and Emergency Management Service of Ecuador (2020) give guidelines for the management of this waste, depending on its place of origin, such as in medical centers, where masks are treated as medical waste to receive adequate treatment, while masks in homes must be disposed of in closed plastic bags and sprayed with disinfectant solutions, which must be placed in another bag and together with ordinary garbage [9]. According to WWF [10], with the incorrect disposal of only 1 % of masks globally, these would be approximately 10 million units or about 40,000 kg of plastic that would pollute the environment, as can already be seen in different places such as beaches, streams, riverbanks, even in the ocean; another problem that these actions entail is the time it takes for this waste to degrade, which can take more than 400 years if they are surgical masks. Unawareness public of the measures contained in the municipal protocol for the prevention of coronavirus contamination results in the disposal of inadequate disposal of masks, gloves, tissues, napkins, and the incorrect storage of suspected or confirmed COVID-19 waste, which raises the risk of contamination for both the public and public servants in the public domain [11]. With this background, it is necessary to implement new environmentally friendly processes and simultaneously obtain products with high potential for use with devolatilization and viable pyrolysis options to valorize these wastes. Regarding incineration actions, it was identified that about 15 thousand tons of COVID-19 test plastic waste had been incinerated globally, while only 494 tons of plastic waste have ended up in landfills. Most countries generate more than 10 tons of plastic waste that is incinerated; furthermore, it was estimated that 99.2 % of the incinerated COVID-19 test plastic waste is composed of polypropylene. Mechanical recycling occupies 16 %, 25 % of the plastic waste is recycled through incineration, and another 40 % is disposed of in landfills, a risk to the environment and wildlife. The remaining 19 % was not appropriately handled and leaked into the environment. It should be noted that open burning and mismanagement of hazardous waste are responsible for specific health problems, including the already confirmed link to cancer, respiratory diseases, and vector-borne diseases [12]. Because these alternatives consist of the thermal and physical-chemical decomposition of organic matter in the absence of an oxidizing medium, significantly reducing the generation of polluting gases and guaranteeing the elimination of dangerous gaseous emissions compared to the direct incineration process. It is conducted under an inert atmosphere using nitrogen, helium, or argon to produce a liquid mixture of hydrocarbons, combustible gases, and solids such as carbon.
Pyrolysis is the first step for combustion and gasification, followed by partial or total oxidation [13].
The pyrolysis process occurs in three stages:
1) Slow decomposition with the production of small amounts of water, carbon dioxide, methane, and hydrogen, products of breaking bonds by the action of high temperatures.
2) Active thermal decomposition begins at approximately 360 °C and ends at 560 °C; deeper fragmentation occurs, and condensable hydrocarbons and tars are generated.
3) Final stage operates at < 600 °C. There is the elimination of hydrogen and other heteroatoms [14].
Pyrolysis is used for treating hospital waste because it does not generate polluting gases such as nitrogen and sulfur oxides, which are produced in incineration, a more economical process; some countries use this technology to dispose of hospital waste [15].
In Ecuador, these studies focused on the recovery of waste in the health field have not been carried out; therefore, in this research work, an option is given for gloves and surgical gowns, whose main components are Natural latex (cis-1,4-polyisoprene) and polypropylene. In addition, contaminating material such as blood is incorporated to analyze the variation it produces in the results.
The determination of a kinetic model for the pyrolysis reaction is proposed in the devolatilization stage due to the technological limitations that imply quantifying the percentage of condensable and no condensable gases and solids as the main products of the pyrolysis reaction. The thermogravimetric analysis evaluates mass losses as a function of temperature at constant heating rates. Subsequently, the activation energy is obtained through kinetic modeling, pre-exponential factor, and reaction order. A model was proposed to achieve a better fit considering a reference temperature. They determine that the presence of blood in the waste causes an increase in activation energy.
Materials and methods
The research analyzed two types of bio-medical waste: surgical gowns and gloves contaminated with human fluids. The procedure for the analysis of these residues is detailed:
Cutting of bio-medical waste: The gowns and the surgical gloves have thermosensitive fabric materials. For this reason, particle size influences the obtaining of mass loss data. Therefore, when operating with tiny particle sizes during the heating escape from the TGA sample holder, for this reason, it was determined that the ideal size to work with is 1x2 cm.
Thermogravimetric Analysis (TGA): This technique, which is widely used for solid materials like polymers, allows the evaluation of the behavior of mass variation as a function of time and temperature in primary decomposition reactions. In addition, it can work on dynamic ramps, where the temperature varies, or in isothermal states. We experimented with temperature ramps for 5, 15, and 15 "Crnin1.
The data provide information on the composition of the material, reaction order, the existence of secondary reactions, and kinetic constants and is currently the most widely used technique to evaluate the energy potential of possible materials that can substitute conventional energy sources. In this case, the lab experiences were using the TGA 1 with the STAR System software, brand METTLER TOLEDO [16], in the Research Area on the Facultad de Ingenieria Quimica-UCE.
Results and discussion
Thermogravimetric analysis
The thermogravimetric analysis used on the devolatilization of the bio-sanitary waste allowed us to obtain the kinetic parameters of the degradation of the waste using three different kinetic models.
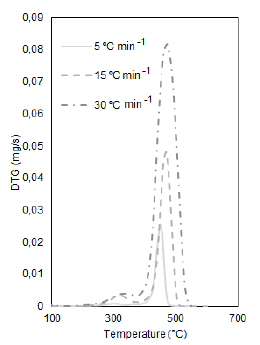
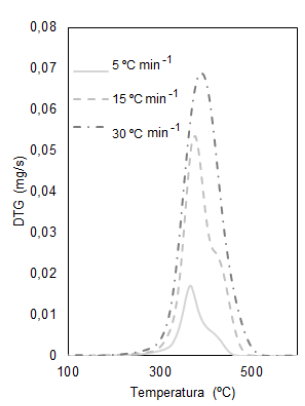
Table 1 shows the maximum temperature in which the mass loss occurs for each heating rate of the bio-sanitary waste, data obtained from the mass loss curves (TG), and those derived from the loss mass (DTG).
Table 1 Time ranges of mass loss.
| Bio-medical waste | Maximum temperatura peaks, °C |
|---|---|
| Clean surgical gowns | 459 |
| Contaminated surgical gowns | 457 |
| Clean surgical gloves | First peak: 375 Second peak: 769 |
| Contaminated surgical gloves | 377 |
It contains all the information about the findings achieved once the statistical methods have been applied (if necessary).
In bio-sanitary waste, the moisture content is not evidenced. The absence of a peak before 100 °C indicates water evaporation and neither peaks belonging to proteins nor low-boiling point monomers. Furthermore, in Figure 1, it is observed that the influence of the heating rates generates a delay in the loss of mass towards higher temperatures.
For gowns (Figure 1), the most significant mass loss is in the temperature range of 220 to 550 °C, representing the decomposition of volatile polypropylene and organic material when registering a shoulder in the main curve. The clean gloves have two peaks: the first in the interval 242 to 517 °C, the most significant mass loss of the polymeric chain of natural rubber or cis-1,4-polyisoprene, and the second peak is in the range of 605 to 748 °C, which due to its high temperature is attributed to the presence of carbonates typical of the powder that internally coats the gloves. For gloves contaminated with blood, their decomposition occurs at 236 to 517 °C, as shown in Figure 2.
It was possible to determine the decomposition capacity of the volatile material belonging to the bio-medical waste analyzed using thermogravimetric analysis as a function of time and temperature data that allowed us to obtain the kinetic parameters. The devolatilization potential is determined with this information.
The experimental methodology is important to highlight that particle size influences obtaining mass loss data. Both surgical gowns and gloves are made with heat-sensitive materials. When operating with very small particle sizes during heating, they escape from the sample holder; for this reason, it was determined that the ideal size to work is 1x2 cm.
Kinetic models
The kinetic modeling of thermo-chemical reactions allows for the establishment of the theoretical behavior of the reaction that is taking place, for which it is necessary to compare the reaction rate defined as the derivative of the conversion as a function of experimental time with that is calculated theoretically with the model. It is required to transform the mass derivative as a time function; in this case, the derivative provided by the thermogravimetry equipment software is taken.
Model 1 - Coats-Redfern [17]: It is an integral method of a non-isoconversional kinetic model, which includes the thermal degradation mechanism, which allows for determining the activation energy and the pre-exponential factor.
Non-isoconversional method: They are those methods where the kinetic parameters are obtained at different conversion values, regardless of the heating rate at which one works.
Model 2 - Friedman [18]: It is a method that compares the loss of mass as a function of the conversion, using different heating rates.
Model 3 - Flynn-Wall-Ozawa (FWO) [19]: It is an integral method that starts from the same concepts as the previous methods and considers the heating rate as a function of time and the Arrhenius equation.
For clean gowns and gloves, follow the decomposition equation:
But in the case of clean gloves, the best adjustment is made by working each peak separately, with its corresponding higher temperature. Thus, in the end, the total dα/dt is the sum of the dα/dt.
A more significant adjustment for contaminated gowns and gloves is achieved by entering a reference temperature belonging to the most significant peak.
Table 2 shows that the calculated kinetic parameters are consistent with those presented by other authors for varied materials or residues with similar compositions; the differences obtained are due to the different non-isoconversional models used that result in different reaction orders.
Table 2 Comparison of the proposed kinetic model by bio-medical waste.
| Author | Bio-medical waste | Material | n | E (kJ.mol1) | A (s-1) |
|---|---|---|---|---|---|
| This investigation | Surgical gowns | Polypropylene | 1 | 232.40 | 3.71x1018 |
| Surgical gloves | Cis-1,4-polyisoprene | 2.5 | 160.54 | 7.8x1010 | |
| Contaminated surgical gowns | Polypropylene + organic matter | 2 | 553.62 | 1.65x10-2 | |
| Contaminated surgical gloves | Cis-1,4-polyisoprene + organic matter | 2 | 152.50 | 3.73x10-3 | |
| Paraschiv, et al. (2015) | Hospital plastics Hospital plastics | Polypropylene Latex | 1 1 | 301.00 148.80 | 3.71x1020 1.74x1011 |
| Cai, et al. (2007) | Recycling of plastics | Polypropylene | 1 | 319.70 | 5.9 |
| Fan, Chen, Huang & Wang (2016) | Latex foam | Latex | 3 | 86.32 | 2.36 x103 |
The conversion data and the inverse of the temperature were adjusted to the linear regression proposed for each model, recording the same reaction order for all samples regardless of the model used to determine the reaction order of each bio-sanitary waste
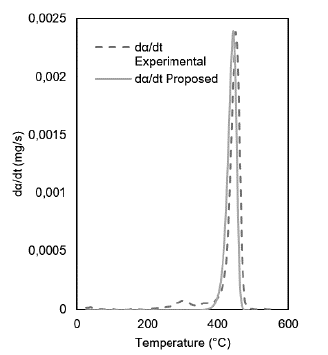
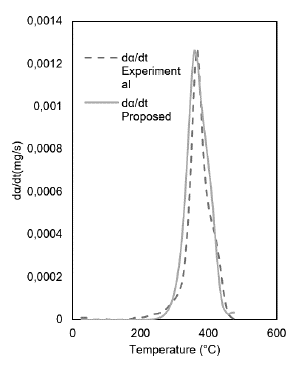
With the results of the best fit models, the model was improved through variations in the activation energy and the pre-exponential factor using the Microsoft Excel calculation tool. The restrictions were: minimizing the objective function (OF) and considering as reference temperature the value corresponding to the majority peak; this last restriction was considered for the contaminated gowns and gloves with an average value of 725.15 and 640.3 °C, respectively. The kinetic model best represents the devolatilization process for clean and contaminated gowns is the Flynn-Wall-Ozawa (FWO) model. In contrast, the Coats-Redfern-based model for clean and contaminated gloves is used.
Statistical analysis
In the experimentation, we evaluated 12 main tests by different heating rates for each of the four bio-sanitary wastes. Each assay was performed in duplicate, as a result, the validation objective. They resulted in a total of 24 experiments. We validated theoretical and experimental data by statistical analysis to And the model that best fits the experimental data concerning the calculated data [20].
The statistical results from the models are found in Tables 3 and 4. In addition, the proposed model is compared with the initial one, thus verifying a significant improvement in the Function Objective (OF) value.
Table 3 Comparison of the results of the statistical analysis for kinetic devolatilization of gowns.
Table 4 Statistical comparison of the kinetic models for surgical gloves.
Through statistical validation, it was verified that there is a significant decrease in the variance values for the proposed model compared to the initial model from 10-6 to 10-8, that is, two orders of magnitude, indicating that there is less data dispersion and that the adjustments achieved are adequate
Regarding the OF function, the average reduction is from 10-6 to 10-7, but for clean gowns, the adjustment is maximum with a 10-12; the curves overlap perfectly.
The critical F value is more significant than F; therefore, the null hypothesis is accepted. The means and standard deviation of the samples' devolatilization velocity values, dα/dt, are equal, indicating that the model raised is like the experimental one.
When optimizing the models, it was obtained that the thermal decomposition or devolatilization reaction follows a second-order behavior for the contaminated coats and gloves. The clean coats it is of the first order. The clean gloves had two peaks and were optimized separately; the first is of the third order. The second is of second order, thus obtaining OF values of 10-6. However, compared with the model's orders, they differ significantly. For example, in the case of the contaminated gowns, the curve obtained by direct adjustment of the kinetic model and the proposed model differ in amplitude, showing an increase in the activation energy from an average value of 63 kJ.mol-1 to 553 kJ.mol-1 and a reaction order from 0,5 to 2. The results of the model fit are presented in Figures 3 and 4.
Determining the kinetic models based on the kinetic parameters that describe the devolatilization of:
Clean gowns:
Contaminated gowns:
Clean gloves:
Contaminated gloves:
Conclusions
The kinetic parameters that describe the devolatilization by thermal degradation of bio sanitary materials have been determined (kinetic equations 4, 5, 6 and 7). For the devolatilization of clean surgical gloves, the conversion rate equation results from the sum of the individual fit of each peak of the dα/dt curve as a function of temperature. For contaminated gowns and gloves, a reference temperature is included in the Arrhenius formula, corresponding to the maximum temperature. The contaminated materials describe the second order's kinetics, the first order's clean gowns, and the gloves. When analyzed by separate peaks, the first peak describes the kinetics of the third order, and the second peak of the second order. Therefore, by future uses in a chemical reactor, clean coats will have a higher conversion rate since the reaction order indicates how sensitive the amount of compound is to the rate at which it is consumed.
The analyzed medical waste determines a specific behavior of the devolatilization process, noting that the activation energy is higher in materials contaminated with blood and impurities compared to clean materials.
For all the samples analyzed, the weight loss is up to 98 %, demonstrating that devolatilization can reduce the initial waste mass and the amount of ash to the maximum (value estimated by TGA), being a viable process to be used in the reduction of hospital waste.