Introduction
Infections are the second cause of death in solid organ transplant patients in the first three years 1. The incidence ranges from 3-50 per 100 patients, depending on the antiviral prophylaxis 1-3. Herpes simplex virus (HSV) type 1 infection is mostly due to viral reactivation (the virus replicates in the oral and genital mucosa and is transported to regional ganglia where it establishes latency), and is rarely due to transmission from the donor to the recipient 2,4,5. One of the causes of HSV type 1 reactivation in transplant patients is treatment with mycophenolate mofetil, muromonab-CD3 (OKT3), alemtuzumab or medications with significant T-cell depletion 4.
The clinical signs of HSV type 1 infection are generally mucocutaneous involvement, herpes labialis; and visceral involvement in the form of hepatitis, esophagitis, pneumonia, corneal blindness, neonatal herpes and, much less frequently, encephalitis. With encephalitis, patients may debut with a seizure syndrome, fever, headache, aphasia, lethargy, confusion and focal deficits which reflect inflammation of the temporal lobe and the orbital surface of the frontal lobe and patients may be left with a permanent seizure disorder, altered consciousness, or motor deficits 2,3,6,7. Ten percent of patients may have gastrointestinal symptoms and an impaired mental status 5.
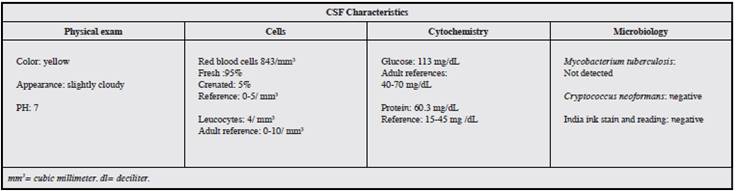
For its neurological diagnosis, cerebrospinal fluid (CSF) analysis, an electroencephalogram (EEG) and neuroimaging may be used. In the first, there may be increased opening pressure and an elevated cell count in more than 90% of patients; pleocytosis is typically predominantly mononuclear, with a mean of 200 cells/mm3, although its absence does not rule out the diagnosis. Proteins are increased and glucose tends to be normal, and culture of the liquid rarely isolates the virus. The gold standard is detection of HSV type 1 DNA by PCR in CSF 2,4,7. The EEG in some patients with HSV shows spike wave and slow wave complexes 2.
In 80% of cases, computerized axial tomography (CAT) shows hypodense lesions in the medial temporal lobe and basal ganglia, edema and mass effect. Magnetic resonance imaging (MRI) has a decreased T1 signal and increased T2 signal in the orbitofrontal and medial temporal lobes 2.
The recommended treatment is IV acyclovir 10 mg/kg every eight hours for 21 days in adult patients 4. In the event of acyclovir resistance, it may be treated with foscarnet; other types of treatment are still under investigation 4.
Clinical case
The patient was a 56-year-old man from Montería, Córdoba, a rancher with a history of chronic kidney disease (CKD) of indeterminate cause and the recipient of a cadaveric kidney transplant in July 2006. The donor had brain death due to head trauma, a negative infection profile, and no serum tests for HSV type 1 or 2, in accordance with the 2004 national policy 8.
Rabbit immunoglobulin was used for induction, and maintenance immunosuppression began at transplantation with tacrolimus XL 5 mg/day, reaching levels between 2.8 ng/mL (the lowest value) and 5.3 (the highest value), with its respective adjustments; and mycophenolic acid 1,440 mg/day (this medication's levels are not monitored in this country). In 2014, he experienced IA acute cellular rejection, according to the 2013 Banff criteria 9, for which he was treated with methylprednisolone and required rabbit immunoglobulin. In August 2016, chronic graft nephropathy was documented.
In September 2018, he was admitted to the emergency room due to a one-week history of exacerbated chronic diarrhea, with 10 liquid stools per day without mucous, afebrile, and no emesis. On a review of systems, he reported losing 4 kg over the previous month.
On physical exam, he had dry oral mucosa, generalized pallor, tachycardia (123 beats/minute), blood pressure (BP) 145/85 mmHG, temperature 36.7°C, BMI 35.71 kg/m2, 96% saturation on 21% FiO2the graft was palpated in the right iliac fossa, and he had no extremity edema. On neurological exam he was alert, conscious, and oriented, with no apparent deficit.
Admission lab exams showed a CBC without leukocytosis; normocytic normochromic anemia; normal potassium, and creatinine at 3.73 mg/dL (baseline creatinine of 1.6 mg/dL). Los paraclínicos de ingreso mostraron hemograma sin leucocitosis, anemia normocítica, normocrómica, potasio normal, creatinina de 3.73 mg/dL (creatinina basal de 1.6 mg/dL).
During the first 24 hours of admission the patient had a tonic-clonic seizure with a prolonged postictal state, stupor, persistent Glasgow 9-10, and required orotracheal intubation, benzodiazepines and broad-spectrum antibiotic treatment (meropenem and vancomycin). Immunosuppression was suspended due to the severity of the clinical picture and intensive care unit (ICU) treatment. In the postictal state, he had a hypertensive crisis, severe metabolic acidosis, hyperlactatemia, elevation of creatinine to 6.03 mg/dL, blood urea nitrogen (BUN) 69.6 mg/dL, and a CBC showing leukocytosis (12,320/mm3), lymphopenia (7.8%) and normal electrolytes. Blood cultures, stool tests and urine culture were all negative. A chest x-ray showed cardiomegaly and an abdominal and pelvic CAT suggested ileus. A viral load test for cytomegalovirus (CMV) was negative and a kidney biopsy showed transplant glomerulopathy, without rejection. The simple head CAT showed a slight heterogeneous increase in bone mass which could be related to hyperparathyroidism. In the subsequent 92 hours, the patient had a torpid clinical course, remaining in status epilepticus despite anticonvulsant treatment and requiring continuous renal replacement therapy.
The differential diagnosis included gut-derived sepsis, neuroinfection, immunosuppressant toxicity (despite the fact that tacrolimus levels were always within normal to low ranges; in this country, we do not have clinical access to mycophenolate levels that might indicate a high dose of the medication) and hypoxic encephalopathy versus structural epilepsy.
A brain MRI showed discrete cortical restriction in the left insula and the posterior third of the ipsilateral cingulategyrus (Figure 1). The proposed differential diagnosis was autoimmune encephalitis, with negative antinuclear, anti-DNA, and extractable antibodies.
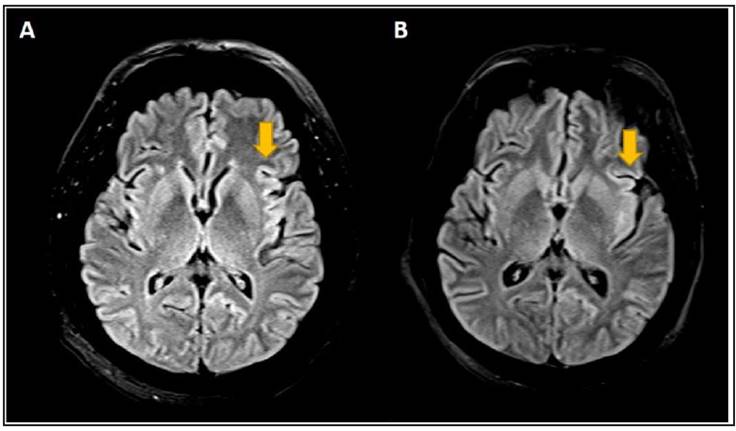
Figure 1 Progression of left insular lesion on brain MRI. Panel A. Discrete cortical restriction and thickening on the diffusion sequence in the left insula and the posterior third of the ipsilateral cingulate gyrus on this case's first brain MRI. Panel B. Increased signal in the left insula and the posterior third of the ipsilateral cingulate gyrus with effacement on a follow-up brain MRI on day 4. Diagnostic Imaging Department.
A lumbar puncture (LP) and CSF analysis led to a suspicion of viral encephalitis, due to the presence of elevated protein and glucose levels and pleocytosis (Table 1). Cultures for mycobacterium and cryptococcus, India ink stain and adenosine deaminase (ADA) levels in CSF were reported as negative.
In the light of persistent neurological impairment, an EEG was performed which registered seizures, and a new LP was performed using CSF FilmArray, which four hours later reported a positive PCR for HSV type 1, consolidating the diagnosis of HSV type 1 encephalitis. Treatment was begun with acyclovir adjusted to the kidney function at 6 mg/kg/ day, for a dose of 600 mg/day IV for 21 days. After seven days of treatment, the patient's neurological symptoms improved. Immunosuppression was restarted, multidisciplinary rehabilitation was performed and, after 21 days of treatment, he was discharged from the hospital.
At follow up 12 weeks after the acute kidney injury, the patient still required hemodialysis. No neurological sequelae were documented, and studies were begun for repeat kidney transplantation.
Discussion
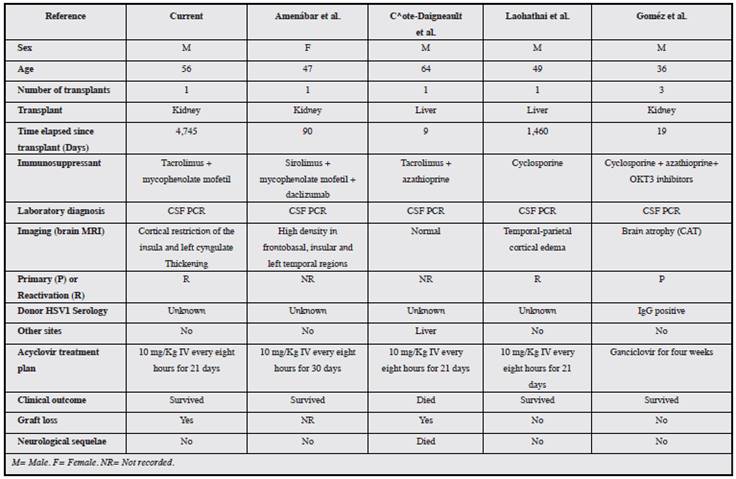
The family of herpes viruses is responsible for most viral infections in transplant patients 6. The estimated incidence of HSV encephalitis is 2-4 cases per year per million, with HSV 1 being responsible for most of these 6. The literature reports four cases of HSV type 1 encephalitis (Table 2): one liver transplant patient with HSV type 1 encephalitis and aspergillosis coinfection, who died 10; and three other patients whose infection remitted with acyclovir treatment, one of whom had a neurological sequela 6,10-12.
Antiviral prophylaxis in transplantation has reduced the incidence of HSV type 1 encephalitis by up to 70%, using ganciclovir and valganciclovir 5. In a meta-analysis which included nine studies with 1,483 transplant patients, the reduction in the incidence of HSV or herpes zoster virus (HZV) infections was significant using valganciclovir or ganciclovir prophylaxis versus placebo 13. The patient in the current case received anti-CMV prophylaxis with valganciclovir for 90 days immediately post-transplant.
Central nervous system diseases associated with the herpes virus include encephalitis/myelitis and post-transplant lymphoproliferative disorder (PTLD). The diagnosis of herpes virus encephalitis according to international criteria includes: 1) neurological signs or symptoms; 2) viral DNA-positive CSF; 3) inflammatory changes on neurological imaging; and 4) lack of other etiological evidence or established diseases 14.
Although HSV type 1 diarrhea is uncommon, mycophenolate has been associated with persistent diarrhea in up to 38.5% of kidney transplant patients. Patients on this immunosuppressant have 1.5 times more risk of gastrointestinal symptoms 15.
Most of the currently available diagnostic methods are inexact, with the exception of CSF PCR, which detects HSV type 1 with a 100% sensitivity, 99.9% specificity, 100% negative predictive value (NPV) and 91.7% positive predictive value (PPV) 2,16,17. Tests such as serology and cultures are often negative and may not be useful for diagnosis 6,10. All the cases consulted were diagnosed by viral detection through CSF PCR 5,10-12.
A very important topic in serious infections, according to theKidney Disease: Improving Global Outcomes(KDIGO) guidelines, is to reduce or withdraw immunosuppression to reduce the risk of disseminated infection, although this may increase the possibility of graft rejection 18,19. This consideration was applied in this patient; however, he did not experience rejection, but neither did he fully recover his kidney function.
There is a worldwide epidemiological transition in HSV type 1 transmission which has been very well documented, especially in Western countries. To this end, the World Health Organization is leading the efforts to accelerate the development of vaccines 3,20. Herpes simplex virus type 1 encephalitis in kidney transplant infections is a viral infection which must be rapidly diagnosed and treated promptly 12. The limitations in early diagnosis of the microbiological agent may cause neurological sequelae and affect the kidney graft 4,12,21,22.
In summary, this is an uncommon case of HSV encephalitis 12 years after transplant which progressed satisfactorily with acyclovir, from a neurological standpoint.











 text in
text in