Introduction
In December 2019, an outbreak of an emergent disease caused by a novel coronavirus (SARS-CoV-2) began in Wuhan, China, which rapidly spread throughout all the con>tinents, and was declared a pandemic on March 12, 2020 1.
Chinese statistics and the disease’s behavior indicate that it has a clinical presentation spectrum ranging from a mild to a serious form (around 80% of those infected having the mild form), with an approximate lethality rate of 2.3%, reaching its highest percentage (14.8%) in those over 80 years old 2,3.
As of July 2020, SARS-CoV-2 infection has affected more than 11 million people worldwide 4,5. Given this significant number of cases at a global level, and consid>ering that this is an emergent virus for which we have no knowledge of effective treatment measures, there is a need for research to find effective options to decrease mortality, the need for ventilatory support, hospital stay, or at least the length of viral excretion which may limit its transmission and expansion 3.
Through clinical trials, Chinese experts proposed chloro>quine, an antimalarial already known to be a pharmacologi>cal option for treating the disease, finding good results with regard to viral elimination compared to control groups. Its use in this disease is based on the fact that this medication has broad-spectrum antiviral activity through increasing the endosomal pH necessary for viral fusion to the cell, and interferes with glycosylation of the SARS-CoV cell recep>tors. The first in vitro studies report that chloroquine blocks SARS-CoV-2 infection at a low micromolar concentration, a maximum effective concentration of 1.13 μM and a semi-cytotoxic concentration greater than 100 μM 6.
The results of more than 100 patients have shown that chloroquine phosphate (CQ) is superior to the control treat>ment in inhibiting the progression of pneumonia, improving lung imaging findings, negativating the virus and shortening the disease course, according to the little available evidence. It is important to note that, to date, no serious adverse reac>tions to chloroquine phosphate have been reported in the previously mentioned patients 6. Hydroxychloroquine (HCQ), a chloroquine analog, has proven to have a higher clinical safety profile, which suggests that it could be a better treatment option 3.
It is important to point out that new data emerge daily on the clinical characteristics and treatment options of CO>VID-19. However, the objective of this exploratory review is to present the information available to date on the use of hydroxychloroquine as a treatment option for patients infected with SARS-CoV-2.
Materials and methods
Study design
A review was carried out following the methodological framework proposed by Arksey and O’Malley. The follow>ing five steps were followed for this exploratory review: a) identification of a clear research objective and search strategy, b) identification of published and unpublished articles, c) selection of articles, d) data extraction and map>ping, and e) summary, discussion, analysis and reporting of the results. The review answered the question: What is the available evidence on the use of hydroxychloroquine as treatment for SARS-CoV-2 infection in the general population?
Inclusion and exclusion criteria
The following inclusion criteria were considered for this article: publications on the use of hydroxychloroquine as monotherapy or in combination for treatment of SARS-CoV-2 infection; articles with their own data, either in vitro o in vivo, or theoretical articles (narrative reviews or letters to the editor); published in English or Spanish, and published between December 1, 2019 and July 2, 2020. In the case of clinical trials, completed and ongoing studies were included.
Articles were excluded if the complete text was not avail>able and if they only spoke of using hydroxychloroquine as prophylaxis in the management of COVID-19.
Literature search strategies
The literature for this review was identified through a search of the following online databases: PubMed, MedLine, Lilacs, Scopus, Clinical Trials, Cochrane and CNKI. The search terms were “hydroxychloroquine” AND “treatment” AND “COVID-19” OR “Coronavirus” OR “SARS-CoV-2”.
Study selection and data extraction
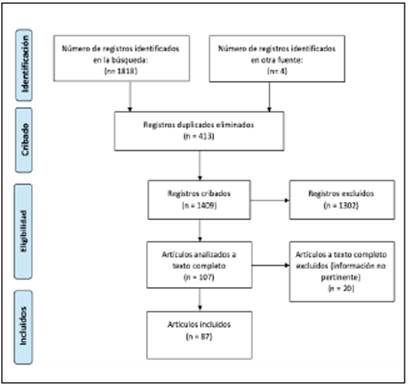
Articles were selected after reviewing their abstracts and determining that they contained the information of interest. At the same time, articles that did not meet the search criteria and duplicate articles were eliminated. In the end, 87 articles were included in this analysis. Figure 1 presents a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the search process and selection of study articles.
After selecting the articles, the data were extracted and recorded on an Excel spreadsheet in which two formats were created, one for recording clinical trials and the other for the remaining documents. The extracted data included date of publication, language of publication, title of the article, country and affiliation of the authors, objectives of the study, the research domain and the key findings. For clinical trials, the recruitment status, study design, sample size, control and beginning or registration date were also recorded.
Summary of findings
Based on the primary objective of the study, the articles were classified as completed and ongoing clinical trials, meta-analyses, systematic reviews, observational studies, reviews and letters to the editor which include studies on hydroxychloroquine as COVID-19 treatment. The dates of publication, journal language, author affiliation, objec>tives of the study, methodological characteristics, where applicable, and results were analyzed.
Results and discussion
Characteristics of the published studies
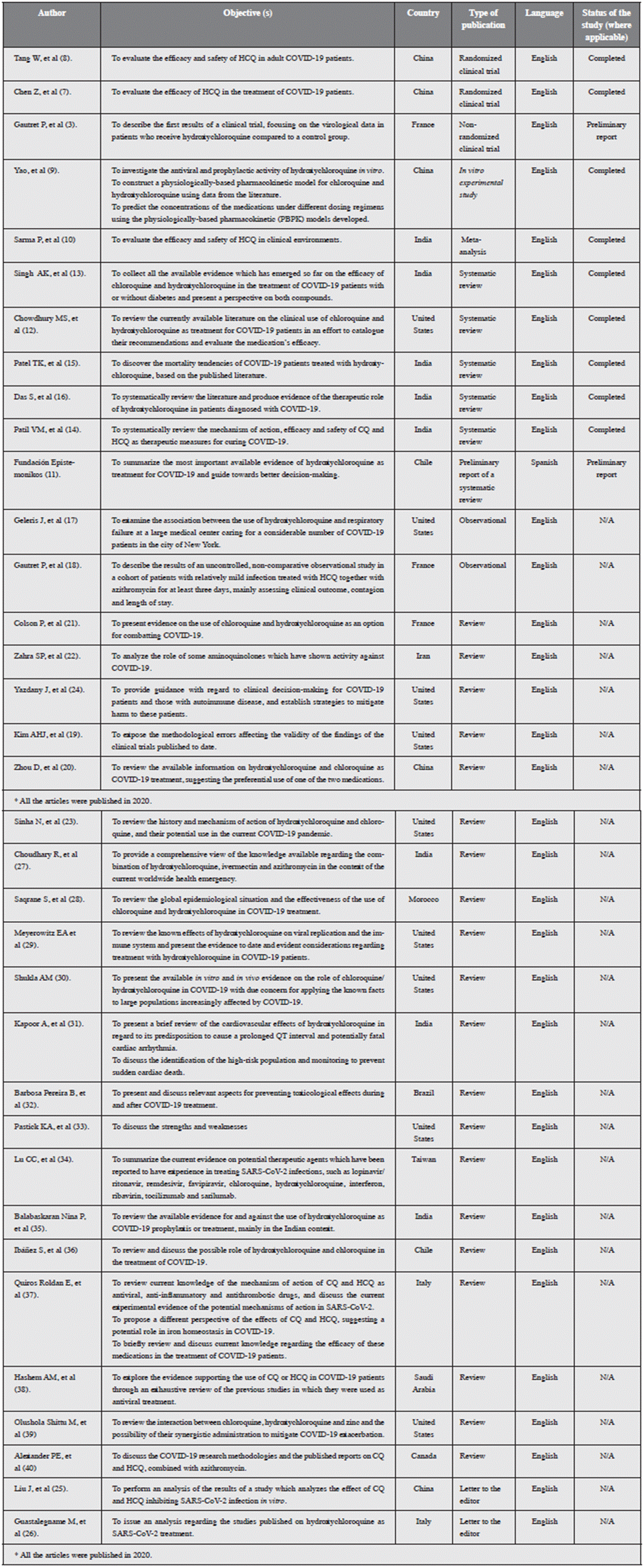
Eighty-seven academic articles were selected, including two randomized clinical trials, one nonrandomized clinical trial, one in vitro experimental study, 52 ongoing clinical tri>als, one meta-analysis, six systematic reviews (one with only a preliminary report), two observational studies, 20 review articles and two letters to the editor, all of which seek to col>lect or create evidence on the use of hydroxychloroquine in COVID-19. All the research studies included in this review are organized in Tables 1 and 2, separating the completed experimental and nonexperimental studies from the ongoing clinical trials, and detailing the main author, objective(s), year, country in which it was carried out, type of publication, language and phase of the study, for experimental studies.
Table 2 General characteristics of the ongoing clinical trials (ClinicalTrials) (N = 52).*
| Author | Objective (s) | Country | Type of publication | Language | Pitase(Where applicable) |
|---|---|---|---|---|---|
| Kongsacngdao S, et al (41). | To compare various combinations of protc:ase inhibitors, osdtamivir, favipravir and hydroxychloroquine for treating COVID-19. | Thailand | Randomized clinical trial | English | Phase 3 |
| Mitja O, et al (42). | To evaluate the efficacy of the "test an.d treat'" strategy in infected patients, and prophylactic treatment with chloroquine for all contacts. | Spain | Randomized clinical trial | English | Phase 3 |
| Domingo P, et al (43). | To evaluate the use of tocilizumab combined with hydroxychloroquine and azithromycin for treating hospitalized adults with COVID-19. | Spain | Randomized clinical trial | English | Phase 2 |
| Hongzhou L, et al (44). | To evaluate the efficacy and safety of HCQ in the treatment of COVID-19 pneumonia. | Cbina | Randomized clinical trial | English | Phase 3 |
| Dubee V, et al (45). | To evaluate the response to treatrnent with hydroxychloroquine in COVID-19 patients in tenns of prognosis, mortality and use ofiMV | France | Randomized clinical trial | English | Phase 3 |
| Zampieri F, et al (46). | To compare HCQ + azithromycin and HCQ monotherapy for treating hospital- ized patients with COVID-19. | Brazil | Randomized clinical trial | English | Phase 3 |
| Amaravadi R, et al (47). | To evaluate, throngh three different cohorts, the use of high-dose HCQ as treat- ment for COVID-19 patients at home, different doses of HCQ in hospitalized patients, and low-dose HCQ as prevention in healthcarc workers. | United States | Randomized clinical trial | English | Phase 2 |
| Brown S, et al (48). | To compare hydroxychloroquine and azithromycin to detennine which is bet- ter for t:reating hospitalized patients with suspected or confinned COVID4 19. | United States | Randomized clinical trial | English | Phase 2 |
| Hemandez C, et al (49). | To evaluate the safety and efficacy of hydroxychloroquine as treatrnent for severe COVID-19 respiratory disease. | Mexico | Randomized clinical trial | English | Phase 3 |
| Farooq U, etal (50). | To find the effectiveness ofhydroxychloroquine as monotherapy and combined with azithromycin in patients withmild to severe COVID-19 pneumonia atAyub Teaching Hospital, Pakistan. | Pakistan | Randomized clinical trial | English | Phase 3 |
| Berwanger O, et al (51). | To evaluare the efficacy and s<t'ety ofhydroxychloroquine combined withazithro- mycin, compared to hydroxychloroquine monotherapy, in patients hospitalized with SARS-CoV-2 pneumonia in Brazil. | Brazil | Randomized clinical trial | English | Phase 3 |
| RambamP,et al (52). | To evaluate the efficacy of HCQ in COVID-19 patients with a recent diagnosis who have mild to moderate disease ora risk for complications. | Israel | Randomized clinical trial | English | Phase 1 |
| Ferrara L, et al (53). | To evaluate the efficacy ofHCQ and azithromycin as treatment for moderate to scvere COVID4 19 pnc:wnonia. | Brazil | Non4 randomizc:d clinical trial | English | Phase 1 |
| Martinelli G, et al (54). | To evaluate the role of hydroxychloroquine versus observation alone in the prevention of COVID-19 infection or the treatment of patients with early-stage COVID-19. | Italy | Randomized clinical trial | English | Phase 2 |
| Novartis Phannaceuticals (55). | To detennine if monotherapy with oral hydroxychloroquine, or in combination with azithromycin, produces a clinical benefit in hospitalized patients with COVID-19 pneumonia. | United States | Randomized clinical trial | English | Phase 3 |
| Nori P, etal | To evaluate the efficacy of hydroxychloroquine in healtheare workers in the Montefiare Healthcare System (United States) who are atgreater riskfor severe COVID-19 disease. | United States | Non- randomized clinical trial | English | Phase 2 |
| Duska F, et al (56) | To test the hypothesis that earlyadministration of combination therapy(hydroxy- chloroquine andazithromycin) slows disease progressionandimproves survival without the need for mechanical ventilation. | Czech Republic | Randontized clinical tria! | English | Phase3 |
| Curlin M, et al (57). | To evaluate the efficacy and safety of hydroxychloroquine as trcatment for patients with lower respiratory tract infections dueto SARS-CoV 2. | United States | Randontized clinical tria! | English | Phase4 |
| Spivak A, et al (58). | To evaluate the efficacy and safety ofhydroxychloroquine in reducing viral load and viral elintination in adult ambulatory COVID-19 patients. | United States | Randontized clinical tria! | English | Phase2 |
| Richards WO, et al (59). | To "'e ifhydroxychloroquine decreases theviralload (throughPCR), seven days after beginning treatment, compared to control patients who receive a placebo. | United States | Randontized clinical tria! | English | Phase2 and3 |
| Mordmüller B, et al (60). | To identify the effect of hydroxychloroquine on in vivo viral clearance . | Germany | Randontized clinical tria! | English | Phase3 |
| Conigliaro J, et al (61). | To evaluate and compare the clinical efficacy of treatment with hydroxychlo- roquine or combine d with high-dose intravenous famotidine, in hospitalized COVID-19 patients. | United States | Randontized clinical tria! | English | Phase3 |
| Papanicolaou G, et al (62). | To test the efficacy ofhydroxychloroquine as treatment for COVID-19 patients. | United States | Randontized clinical tria! | English | Phase2 |
| Sanofi(63). | To evaluate the effect of hydroxychloroquine on the nasopharyngeal viral load of ambulatory SARS-CoV-2 patients. | United States | Randontized clinical tria! | English | Phase 1 |
| Shah P, et al (64). | To evaluate the efficacy of combination therapy with azithromycin, hydroxy+ chloroquine and zinc or favipiravir in patients with suspectc:d or confinned COVID-19 infection. | England | Randontized clinical tria! | English | Phase3 |
| Servolo de Medeiros E, et al (65). | To evaluate the safety and efficacy of hydroxychloroquine in patients with symptomatic SARS-CoV-2. | United States | Randontized clinical tria! | English | Phase3 |
| Thakore A, et al (66). | To evaluate the safety and efficacy of hydroxychloroquine and zinc combined with azithromycin or doxycycline in a high-risk COVID+19-positive ambula+ tory population. | United States | Randontized clinical tria! | English | Phase4 |
| Reyne s J, et al (67). | To evaluate the efficacy and safety of hydroxychloroquine combined with azithromycin compared to hydroxychloroquine monotherapy in hospitalized patients with confinned COVID-19 pneumonia. | France | Randontized clinical tria! | English | Phase2 and3 |
| \<>jta D, et al (68). | To prove that high-dose hydroxychloroquine for two weeks maybe an effective medication for both ambulatory patient treatmcnt as wc:ll as prophylaxisltreat- ment for healthcare workers. | United States | Randontized clinical tria! | English | Phase2 |
| O'Hal loran J,et al (69). | To test the efficacy and recovery time of non-critica! (not requiring mechanical ventilation) hospitalized patients with COVID-19 who will receive hydroxy- chloroquine or chloroquine with or without azithromycin. | United States | Randontized clinical tria! | English | Phase3 |
| Akram J,et al (70). | To evaluate the efficacy of hydroxychloroquine in eliminating the virus and improving the course of the disease, oompared to other intetventions: oseltamivir and azithromycin alone and combined with hydroxychloroquine. | Pakistan | Randontized clinical tria! | English | Phase3 |
| Thompson B, et al (71). | To evaluate the efficacy ofhydroxychloroquine for treating hospitalized adults with eOVID-19. | United States | Randontized clinical tria! | English | Phase 3 |
| Abd-Elsalamb S, et al (72). | To rescarch if zinc supplemcnt:zbon improvc:s thc clinical efficacyof chloroquine in eOVID-19 treatment. | Fgypt | Randontized clinical tria! | English | Phase 3 |
| Esmat G, et al (73). | To evaluate the safety and efficacy of adding anti-hepatitis e (HCV) treatment to the standard regimen for treating patients who are candidates for hydroxy+ chloroquine according to the Fgyptian MOHP protocol. | Fgypt | Randontized clinical tria! | English | Phase 2 and3 |
| Suputtamongkol Y, etal (74). | To evaluate oral ivennectin versus hydroxychloroquine plus darunavirJritonavir in adult asymptomatic SARS-CoV-2 carriers in the 1hai population. | Thailand | Randontized clinical tria! | English | Phase 4 |
| WellStar Health System (75). | To evaluate thc impact of hydroxychloroquine on hospitalized patients with COVID -19 and risk factors for critical/severe disease. | United States | Randontized clinical tria! | English | Phase 4 |
| Rea-Neto A, et al (76). | To test if chloroquine or hydroxychloroquire are effective in t:reating COVID-19 and improving a primary ordinal outcome composed of a nine-level scale recommended by WHO. | Brazil | Randontized clinical tria! | English | Phase 3 |
| Letaief A, et al (77). | To investigate the efficacy and tolerance of a five-day regirnen of hydroxy- chloroquine or hydroxychloroquine and azithromycin in COVID·19 patients. | Tunisia | Randontized clinical tria! | English | Phase 3 |
| KaraA, etal (78). | To evaluate the efficacy and safety ofhydroxychloroquine and favipiravir in the treatment of patients with possible or confinned eOVID-19. | Turkey | Randontized clinical tria! | English | Phase 3 |
| Reis G, etal (79). | To evaluate the use of hydroxychloroquine and lopinavir/ritonavir alone or combined in COVID-19 patients. | Brazil | Randontized clinical tria! | English | Phase 3 |
| Ried K, et al (80). | To evaluate the efficacy and safety of azithromycin, hydroxychloroquine, zinc, vitantin D3/B12 and vitantin e treatment compared to azithromycin, hydroxy- chloroquine, zinc, and vitantin D3/B12 in participants with eOVID-19. | Australia | Randontized clinical tria! | English | Phase 2 |
| Bosaeed M, et al (81). | To evaluate the efficacy of the combination offavipiravirand hydroxychloroquine as a potencial treatment for moderate to severe COVID-19 cases. | Saudi Arabia | Randontized clinical tria! | English | NIA |
| Sartori V, et al (82). | To evaluate the efficacy of a combination of hydroxychloroquine and azithro- mycin in the fall in viral load at day five in patients with eOVID-19 and hematologic malignancies. | France | Randontized clinical tria! | English | Phase 2 |
| Arreola Guerra JM,et al (83). | To evaluate the safety and efficacy of treatment with hydroxychloroquine and ivennectinfor serious COVID-19 infections in oon-critical hospitalized patients. | Mexico | Randontized clinical tria! | English | Phase 3 |
| Lutfy S, et al (84). | To investigatc the possible beneficia!effects ofhydroxychloroquine in the trcat- ment of eOVID-19 patients. | Saudi Arabia | Randontized clinical tria! | English | Phase 2 |
| Gabrielli A, et al (85). | To evaluate if the additionof tofacitinib to the standard hydroxychloroquine treat- ment in the earlyphase of COVID-19 pnemnonitis can prevent the development of severe respiratory failure requiring mechanical ventilation. | Ita!y | Randontized clinical tria! | English | Phase 2 |
| Taieb F, et al (86). | To cvaluate and compare viral clearance between thc differcnt therapeutic interventions: hydroxychloroquine and the combinationofhydroxycWoroquine and azithromycin. | Senegal | Randontized clinical tria! | English | Phase 3 |
| Al Qahtani M, et al (87). | To compare three anns: hydroxychloroquine, favipiravir, and supportive care alone, in symptomatic SARS-CoV-2 patients. | Bahrain | Randomized clinical trial | English | Phase2 and3 |
| Cheng SH, et al (88). | To evaluate the efficacy and tolerability of hydroxychloroquine sulfate in adult patients with mild to moderate COVID-19, compared to the standard treatment. | Taiwan | Randomized clinical trial | English | Phase4 |
| Genton B, et al (89). | To evaluate the efficacy of early treatment with hydroxychloroquine in ambula- tory COVID419 patients to reduce the incidence and severityof complications, in- cluding secondary hospitalization, ICU admission, pulmonary disease and death. | Switzerland | Randomized clinical trial | English | Phase2 and3 |
| HorbyPW, et al (90). | To investigate if treatment with lopinavir-ritonavir, hydroxychloroquine, cor- ticosteroids, azithromycin, convalescent plasma or tocilizmnab prevents death in COVID-19 patients. | England | Randomized clinical trial | English | Phase2 and3 |
| Cook S, et al (91). | To evaluate the use ofhydroxychloroquine inmoderately ill hospitalized patients with SARS-CoV-2 pneumonia. | United States | Randomized clinical trial | English | Phase4 |
| * All the articles were published in 2020. | |||||
All the articles were published in 2020, since the pan>demic began during the last days of 2019. The countries where the articles were most often conducted and/or where the most clinical trials are ongoing were United States, India and Brazil, making up almost 46% of the reviewed articles, highlighting the United States with almost 30%. Other countries with publications were China and France, with 7% each; Italy with 4.5%; Saudi Arabia with a little more than 3%; Chile, Taiwan, Thailand, Spain, Mexico, Pakistan, England and Egypt with 2.2%; and Iran, Morocco, Canada, Israel, Czech Republic, Germany, Tunisia, Turkey, Australia, Senegal, Bahrain and Switzerland with only 1.1% of the reviewed articles. All the articles were in English, except for one which was in Spanish. Most of the articles were published in infectology journals along with internal medicine, molecular biology, chemistry, emergency medi>cine and bioscience, among others, showing the interest of various areas in researching this topic.
With regard to the four experimental studies reviewed, these draw attention to the lack of available evidence regard>ing the use of HCQ as a treatment for COVID-19, which is understandable in the context of an emergent disease.
We only had two randomized clinical trials, one pub>lished by Chen Z, et al. 7, with final results available, which evaluates the effectiveness of hydroxychloroquine treatment. This study, despite results suggesting a favorable result of hydroxychloroquine use, has low evidence in this regard due to some biases such as measurement (as there is no clear information on the randomization process) and concept bias (due to a short follow up time), along with a shaky statistical analysis in which some of the possible confounding variables are not mentioned, considering the inclusion and exclusion criteria used. The other random>ized clinical trial is the one published by Tan W, et al. 8 with a sample of 150 patients, which is greater than that used by Chen Z, and the results are less encouraging than those of the other study, since this one could not show a greater probability of negativizing the SARS-CoV-2 test with hydroxychloroquine, and there was a greater number of adverse events in the group receiving the antimalarial.
A preliminary report of a nonrandomized clinical trial published by Gautret P, et al. 3 was found in the literature, which has several methodological flaws inherent in this type of study, as well as a very small sample size. Thus, it does not contribute results with sufficient validity.
Another experimental study included in the review was that of Yao, et al. 9. This was an in vitro study which supports the role of hydroxychloroquine in SARS-CoV-2 infection, opening the debate on its effectiveness; however, these findings must be confirmed with clinical studies.
Of the 31 non-experimental studies reviewed, we high>light one meta-analysis, six systematic reviews and two observational studies. The meta-analysis concludes that treatment with hydroxychloroquine may be beneficial in terms of radiological progression, with a safety profile comparable to the control treatment. In addition, it mentions the possible benefits with regard to temperature normaliza>tion time and the resolution of symptoms such as cough. However, no significant differences were found in terms of virological cure, death or worsening disease; it must be taken into account that the number of clinical studies analyzed was small, with a small number of participants 10. With regard to the systematic reviews, most coincide in the lack of available evidence on the use of hydroxychloroquine and highlight the multiple limitations of the clinical trials published to date. However, they propose its use in the context of the pandemic, in the absence of any other valid treatment option, considering its risk-benefit 11-14. One of these reviews also highlights the need to monitor the risk (in patients who receive it) of adverse effects, keeping in mind aspects such as prior use of the medication, risk of retinopathy, and cardiovascular diseases, among others 14. The systematic reviews by Patel T, et al. and Das S, et al. 15,16 do not recommend the use of hydroxychloroquine in COVID-19 patients. The first concludes that hydroxy>chloroquine does not improve mortality and, if given along with azithromycin, increases the risk of mortality compared to those who do not receive either of these medications. The second argues that the results of this medication are unsatisfactory, although the methodological flaws of the studies must be taken into account. One observational study by Gautret, et al. 18 studied a cohort of 80 patients treated with combined hydroxychloroquine and azithromycin for a minimum of three days. All the patients improved clinically, and a decreased nasopharyngeal viral load was demonstrated on days seven and eight; however, the sample size, short follow-up time and an apparent selection bias with patients having a lower NEWS scale, limit the validity of the study. On the other hand, the observational study published most recently by Geleris, et al. 17 is one of the studies with one of the largest samples (n=1,446). In its conclusions, no as>sociation was found between the use of hydroxychloroquine and the outcomes of intubation or death, although, being an observational study, confounding factors and biases were not measured. Both observational studies coincide in the need to create clinical trials which will produce final results with regard to the use of the antimalarial as a COVID-19 treat>ment 17. These results coincide with what was published in the reviews by Kim AHJ, et al. and Zhou D, et al. 19,20
Some of the review articles analyzed mention that hy>droxychloroquine causes a similar effect on the viruses as chloroquine, since they share the same mechanism of ac>tion, and that hydroxychloroquine may be a better option for treating SARS-CoV-2 since it has fewer adverse effects, making it safer to use 20-23.
Yazdany J, et al. mentioned the public health crisis which could occur in hydroxychloroquine-dependent autoimmune patients due to scarcity of the medication caused by an exag>geration of the available data. They recommend avoiding the misue of hydroxychloroquine until solid scientific evidence becomes available 24.
Finally, two letters to the editor were reviewed. The one by Liu J, et al. 25 suggested that hydroxychloroquine shows its in vitro effect due to its antiviral and anti-inflam>matory action, diminishing the production of cytokines and proinflammatory factors. According to Guastalegname M, et al. 26, the effect of hydroxychloroquine in humans or in vivo SARS-CoV models has not been proven, and thus it may not be useful in COVID-19 patients, and may even cause damaging effects such as those produced in patients with chikungunya infection who received this medication; it must be used with caution.
Limitations
As this review was carried out in a short period of time, seeking to obtain results in a hurry, the literature search was performed by the authors and was not guided by a librarian. Only clinical trials registered on the Clinical Tri>als platform were included; however, this platform has the highest number of records on this topic. Studies published in languages other than English or Spanish were also not included, which may have left some articles out, but it must be noted that most of the articles available in the literature are in English, which ensures having achieved the greatest collection of articles on this topic.
The findings are subject to the scant information avail>able on the topic and the design of the reviewed literature.
Conclusions
The current global situation due to the novel coronavirus SARS-CoV-2 pandemic, its rapid expansion, its exponential growth of infected individuals and the resulting healthcare systems’ crisis necessitates the rapid identification of a cost-effective treatment.
Antimalarials have been proposed as a possible treat>ment for COVID-19 (especially hydroxychloroquine, due to its lower rate of adverse effects compared to chloroquine [20]) because of their known antiviral effect due to their mechanisms of action.
After conducting an exploratory review of the available information to date on this topic, we found that there are two randomized studies with methodological flaws and mutual contradictions. The available clinical trials are mostly nonrandomized, which limits their validity. There are 52 randomized trials currently underway and projected to have results in an average of one year. These intend to test hydroxychloroquine as monotherapy or associated with other antivirals, from which results are expected to be extrapolatable to the global population and to instate hydroxychloroquine as an effective treatment for SARS-CoV-2 infection.
The foregoing concludes that there is no scientific infor>mation available to date that supports and provides enough evidence that hydroxychloroquine may be used to manage the current pandemic. On the contrary, its misuse may re>sult in greater adverse effects for those who take it without an indication, as well as for the health of patients who are dependent on this medication for their survival, as a result of diminished available reserves 24.
For now, we should continue to wait for the results of the ongoing clinical trials in order to determine with enough cer>tainty that this antimalarial should be used, or, on the contrary, rule it out. Overestimation of the information available to date should be avoided and, given the uncertainty regarding its usefulness and potential toxicity, its use in patients should be restricted to clinical studies only.











 text in
text in