Introduction
Bilateral sampling of the petrosal sinuses is the most effective procedure in the differential diagnosis of pituitary or ectopic hypercortisolism, with a sensitivity between 88 and 100% and a specificity of 67-100%. Despite being an invasive procedure, complications are extremely rare, making it a highly useful procedure in clinical practice as part of the diagnostic algorithm of patients with ACTH-dependent Cushing's syndrome. The first samples were taken in the 70s 1,2. Initially, it was performed unilaterally through jugular access to differentiate between pituitary and ectopic etiologies 3. Most endogenous cases are caused by pituitary aderenocorticotropic hormone (ACTH) secreting tumors, which may be surgically resected as a cure 4. Non-invasive imaging and laboratory tests have a low sensitivity for diagnosis. Bilateral inferior petrosal sinus sampling (BIPSS) is very sensitive and specific, and is considered to be the gold standard for diagnosing Cushing's disease 5.
Venous sampling differentiates a pituitary source of cortisol from an ectopic source, since petrosal concentrations of the hormone are expected to be higher than peripheral levels, as it mixes with systemically circulating blood. Since ACTH secretion is intermittent, corticotropin releasing hormone (CRH) (or, alternatively, desmopressin) is administered to stimulate ACTH production and increase the study's sensitivity 6.
Dominance of one petrosal sinus over the other has been shown in some patients; to avoid confusion in interpreting the results, both petrosal sinuses should be sampled to avoid an incorrect identification of the side with a greater concentration. This is why the sample should be taken simultaneously after administering the vasopressin (in our cases we use the desmopressin synthetic analogue) and be comparable for identification to locate the ACTH-secreting tumor 7,8.
Indications
This procedure is reserved for patients with biochemical evidence of ACTH-dependent hypercortisolism who have a magnetic resonance with no demonstrable lesion, or a lesion smaller than 6 mm, and who have hypercortisolism at the time of the procedure 8. It is also indicated in cases that do not respond to hormone tests and in those with a discrepancy between the biochemical findings and imaging studies 9,10.
It is indicated in post-hypophysectomy patients with persistent Cushing's syndrome 11.
Magnetic resonance only detects 50-60% of microadenomas with Cushing's syndrome. Therefore, this procedure should also be considered when no other cause of Cushing' s syndrome is found 12.
Finally, although we do not routinely carry out a CRH test and it is not a part of the flowchart in our setting, according to some authors, BIPSS should be performed on all patients with an ACTH/cortisol response to the CRH test which is inconsistent with Cushing's disease, regardless of the response to the high-dose dexamethasone suppression test, unless magnetic resonance shows evidence of a pituitary adenoma 13.
Anatomical considerations
The venous drainage of the anterior lobe of the pituitary is directed towards hypophyseal veins which lead into a venous network that drains into the cavernous sinus and, subsequently, into the inferior petrosal sinus which flows posteriorly and caudally, ultimately draining into the jugular bulb at the base of the skull 14.
Adrenocorticotropic hormone is produced by anterior pituitary, or adenohypophyseal, basophils in response to CRH secreted by the hypothalamus. Adenomas have hypo-thalamic dysregulation with excessive hormone production which leads to hyperplasia of the secretory cells. Thus, direct measurement of ACTH levels through selective catheterization is relevant for documenting increased production due to hyperplasia of these cells, since the pituitary drains to the petrosal sinuses through the cavernous sinus. It is important to recognize that there may be variants of this drainage. There may not be an inferior petrosal sinus, with drainage going to the vertebral venous sinus, and there may also be plexiform and hypoplastic venous drainage 15. These variants may produce typically lower levels than expected due to the asymmetrical drainage, and may generate false negatives. For this reason, an anatomic venous study should be considered during the venography and included in the report.
Materials and methods
We performed a retrospective study of the technical aspects of petrosal sinus venous sampling from January 1, 2012 to December 31, 2018 at the Hospital Universitario San Vicente Fundación in Medellín, Colombia. The registry included variables such as cortisol levels prior to the procedure, and basal ACTH levels at three, five and 10 minutes after stimulation. We analyzed several variables such as technique and the venographic characteristics of the petrosal sinuses. In addition, in most cases, inferior right and left petrosal sinus prolactin and a basal peripheral sample were recorded for successful verification of petrosal sinus catheterization.
Description of the technique
The procedures were carried out under general anesthesia, early in the morning (from 7:00-9:00 AM). A bilateral trans-femoral venous access was employed using an 18G (Terumo) angiographic needle and positioning short vascular introducers: 5 Fr. (Boston Scientific) on the left and 6 Fr on the right. Subsequently, an intravenous heparin dose (70 IU/kg) was administered with an additional dose if the procedure lasted more than one hour. Under fluoroscopic guidance, 5 Fr or 4 Fr (Terumo) hydrophilic vertebral catheters were advanced bilaterally to the internal jugular veins. Digital subtraction venography was performed to verify catheter positioning (Figure 1a). Using a coaxial and "road map" technique (Figure 1b), 2.8 Fr Progreat (Terumo) catheters were simultaneously advanced when catheterization with hydrophilic vertebral catheters alone was not possible, navigating as close as possible to the cavernous sinus through the petrosal sinus (Figure 1c) before taking the sample. In some cases, a 5 Fr Chaperon (Microvention) guiding catheter was used coaxially with a 4 Fr hydrophilic catheter. Ultraselective phlebography (Figure 1d) was performed, which helps show the contralateral inferior petrosal sinus and serves as a guide for contralateral microcatheterization (Figure 2a).

Figure 1a Phlebography is performed with a 5 Fr vertebral catheter, showing the sigmoid sinus and right internal jugular vein to which one plexiform inferior petrosal sinus drains.

Figure 1b Road map technique, which acts as a guide to visualize the vessels as the sinus is catheterized with the microguide and microcatheter.

Figure 1c The inferior petrosal sinus is selectively microcatheterized with 2.8 Fr Progreat, and another phlebography is performed to verify positioning.

Figure 1d Selective phlebography of the left inferior petrosal sinus is performed showing the coronary sinus and, contralaterally, the petrosal sinus. This technique serves to guide the catheterization of the contralateral sinus.
Once phlebography confirmed appropriate positioning of the catheters (Figure 2b), baseline samples were taken from both petrosal sinuses and from the right femoral access site. Femoral access catheterization was not possible in two cases; left basilic vein access was used in one case, and left jugular access in the other, with 5 Fr (Boston) introducers. After the baseline samples were obtained, a 10.5 µg bolus of desmopressin (diluting a 15 µg/mL ampule from 1 cc to 10 cc and administering 7 cc intravenously) was injected through the peripheral access, in place of CRH, due to availability and costs. Following this, samples were taken from the femoral, basilic or jugular access and from both petrosal sinuses 3, 5 and 10 minutes after the stimulation. The samples were immediately placed in previously marked EDTA-containing tubes which had been previously marked and separated by sampling times, to facilitate safe sampling and packaging to avoid mixing up the samples. They were then transported to the laboratory through a cold chain for safe and effective processing the same day of the sampling.

Figure 2b Right petrosal sinus phlebography confirming adequate positioning of both microcatheters; sampling was subsequently performed at 3, 5 and 10 minutes.
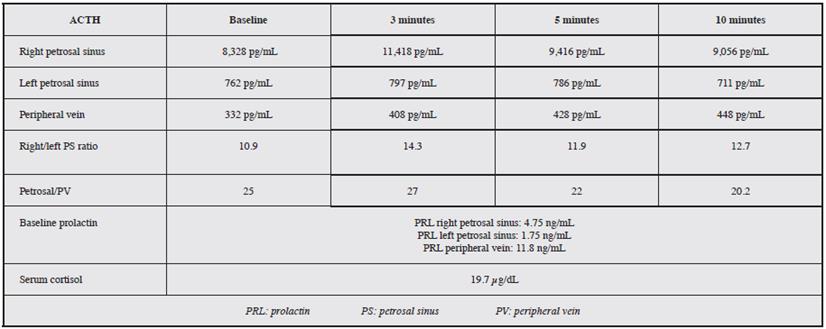
The following parameters were considered as diagnostic criteria:
A central/peripheral ACTH ratio greater than or equal to 2 in the baseline samples.
A central/peripheral ACTH ratio greater than or equal to 3 in any of the subsequent samples after stimulation. If these criteria were not met, it was considered to be ectopic ACTH secretion.
With regard to lateralization, the following was considered:
Results
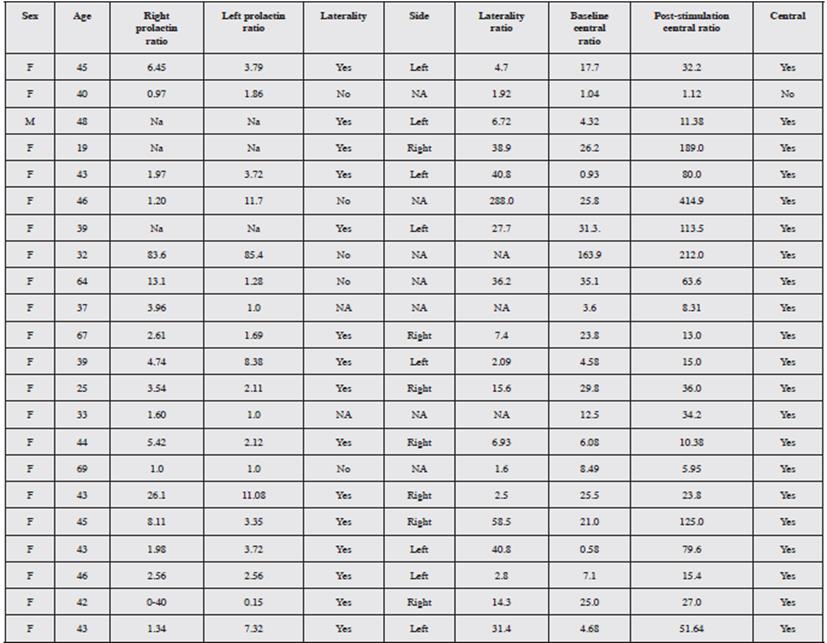
The procedure was performed on an outpatient basis in all cases, with no immediate complications recorded. All the procedures were carried out under general anesthesia and over a period of seven years: 22 selective catheterizations of the petrosal sinuses to determine ACTH levels. A central source was proven in 95.4% of cases. Of these 22 cases, 95.4% were female and 4.6% were male (Figure 3). Table 1 gives an example of the interpretation of the test results, and Table 2 shows the results. The average age was 43.3 years, with a range from 19 to 69 years. An intersinus ratio greater than 1.4 to prove laterality was shown in 68.2% of cases. In the cases in which laterality was proven, there was a tendency towards left laterality in 53.3% of the results. The mean laterality ratio was 32.7, with a range of 1.6-288.0.
The baseline central/peripheral ratio by which 95.4% of the cases were proven was 21.7, on average, and 70.8 after stimulation. The diagnosis was able to be reached with the baseline samples alone in 17 patients (77.3%), and in 21 patients (95.4%) after stimulation, which means that 18.1% more patients with central production were diagnosed following desmopressin administration. In only one case was central production not able to be proven by this method, and in another case, the laboratory did not specify the laterality of the sample, but did prove the central source.
In our results, where the central source was proven, the values obtained at 3, 5 and 10 minutes following stimulation showed a ratio greater than 3.0 in all cases.
Interpretation
The baseline central/peripheral ACTH ratio is greater than 2, and after stimulation with 10.5 µg of desmopressin it is greater than 3, which supports the idea that the source of production is the pituitary.
This study, according to the comparative results of the right vs. left petrosal sinus suggests right lateralization of the ACTH production source.
All the cases were done using only venous access, except for one case in which diagnostic arteriography was performed to adequately identify the petrosal sinuses. Altogether, 90.9% of the cases (20 patients) were done exclusively through femoral venous access; in one case, left basilic vein access was needed and in another case left jugular access was needed, due to a more favorable anatomy for the catheterization.
Discussion
Under normal conditions, the hypothalamus secretes corticotropic hormone which stimulates ACTH secretion in the pituitary, which, in turn, stimulates cortisol secretion from the adrenal glands, regulated by a feedback system. However, some pituitary adenomas secrete ACTH autonomously, causing symptoms of hypercortisolism 16.
The pituitary venous drainage is usually unilateral, despite the existing cavernous interconnections. This makes bilateral venous sampling necessary, to avoid false negatives 17.
The inferior petrosal sinus drains to the jugular bulb, on the inferior anterior border of the jugular foramen, just 6 mm below the foramen; however, this may vary from person to person. The diameter of the inferior petrosal sinus ranges from 2-4 mm at this point, and, in turn, this sinus is the best suited, technically, to microcatheter catheterization, as the hormone flow towards other veins is minimal 18.
Cushing's syndrome is an endocrine disorder involving the hypothalamic-pituitary-adrenal system which leads to excessive cortisol in the blood. Symptoms include central obesity, moon face, proximal muscle weakness, skin changes, hirsutism, arterial hypertension, hyperglycemia, menstrual irregularities and impotence 19.
Cushing's syndrome is caused mainly by exogenous corticoid consumption. However, tumor secretion is the main cause of endogenous hypercortisolism, with a pituitary source being the main etiology in approximately 67% of cases 20,21.
Within the diagnostic approach to Cushing's syndrome, cortisol levels should be measured mainly through urine free cortisol, nocturnal salivary cortisol and the low-dose dexamethasone suppression test 22.
Adrenocorticotropic hormone is a polypeptide hormone produced by the pituitary, which stimulates the adrenal glands. It acts on the adrenal cortex, stimulating steroid genesis, growth of the adrenal cortex and corticosteroid secretion 19.
Its secretion is regulated by corticotropin releasing hormone produced by the hypothalamus; it is pulsatile and has a characteristic circadian rhythm, with maximal secretion in the morning. Its secretion also increases in response to low levels of circulating cortisol, as well as to stress, fever, acute hypoglycemia and major surgeries 19.
This hormone has a short and unstable half-life, is used in corticoid treatments and is very vulnerable to cellular enzymes. It is measured by immunoanalysis, and its synthetic derivative (ACTH 1-24) is used as a pharmacological stimulus to study hypothalamic-pituitary-adrenal function 20.
To diagnose Cushing's syndrome, elevated cortisol must be proven with at least two confirmatory tests. Of all these tests, urinary free cortisol is the most effective method for showing cortisol hypersecretion. A possible diagnostic test for excessive ACTH secretion is the dexamethasone suppression test (dexamethasone is a highly potent synthetic glucocorticoid). This test suppresses pituitary ACTH secretion through negative counterregulation of the hypothalamic-pituitary axis by corticoids. In normal patients, cortisol decreases to less than 5 µg/dL, while in patients with Cushing's syndrome it remains over 10 µg/dL 20.
For its part, desmopressin is a synthetic medication with a similar effect to vasopressin, differing in that it does not act on the V1 receptors responsible for vasoconstriction. Its effect on V2 receptors in the renal collecting tubule after intravenous administration increases the reabsorption of water through aquaporin channel translocation. It also acts on the same V2 receptor of ACTH-producing tumors, stimulating ACTH secretion.
When hypercortisolism has been proven, it should be determined if it is ACTH dependent or not; ACTH blood levels are measured and, if they are low, it is considered to be non-ACTH dependent and a peripheral cause must be sought using abdominal imaging studies 23. On the other hand, if the levels are normal or high, it is considered to be ACTH dependent and is mainly due to secreting tumors, the most frequent being pituitary adenomas and, less frequently, ectopic forms such as gastrinomas, neuroendocrine and carcinoid tumors, pheochromocytomas, and medullary thyroid, pancreatic and pulmonary carcinomas 24.
Non-invasive tests include high-dose (8 mg) dexamethasone tests, which, when they suppress cortisol by more than 50% of the basal value lend support to the source being central or peripheral with a 60% sensitivity and 80% specificity 23.
Magnetic resonance is better than tomography in detecting pituitary tumors, with close to 80% sensitivity; however, very small lesions (smaller than 6 mm) can lead to false negatives 25. Only lesions detected by resonance and positive laboratory parameters confirm Cushing's disease, always keeping in mind that approximately 10-20% of pituitary incidentalomas are non-functioning 26.
According to Oldfield's criteria 27, a central/peripheral ACTH ratio greater than 2.0 at baseline or a ratio over 3.0 after pharmacological stimulation are suggestive of Cushing's disease. Some authors suggest other cut-off values, such as 1.7 28. If these criteria are not met, a peripheral source of ACTH should be sought. With regard to lateralization, an intersinus ratio greater than 1.4 predicts the location of the adenoma on the side with the highest concentration; this does not vary with CRH stimulation 27,29.
In the longest published studies on bilateral venous sampling, the results show a sensitivity between 88 and 100% and a specificity of 67-100% 30-32.
Some authors use diagnostic arteriography as a mapping method for catheterizing the petrosal sinuses 30-32. However, we opted to perform it only when catheterization using exclusively venous mapping was not possible. In our results, we were able to prove the central source of ACTH production in 91.3% of cases, which correlates with the results in the literature 30-32. In our results, the 3, 5 and 10-minute samples were positive in 100% of cases, despite the fact that the authors have described sampling at 1, 3, 5, 10 and 15 minutes (27), and others up to 10 minutes 30-32, which questions the need for sampling at multiple times after stimulation, or if only one post-stimulation sample is necessary to confirm the diagnosis. Successful catheterization was achieved in a high percentage of patients and the discrepant results are probably due to anatomic variants of the pituitary gland's venous drainage, which include hypolastic/aplastic or plexiform sinuses; little initial experience; or difficulty in placing the catheter. Shiu et al. described the anatomy of the petrosal sinuses based on their experience 33, classifying the sinus drainage in four types: type I (25% prevalence), a single petrosal sinus with hypoplasia or absence of the anterior condylar vein; type II (45% prevalence), the anterior condylar vein has the same caliber as the petrosal sinus; type III, the sinus is plexiform (30% prevalence); type IV sinus aplasia (less than 1%); and type V, drainage is lower than usual. In addition, tumors have been described which have cyclical ACTH secretion and do not respond appropriately to pharmacological stimulation, as a smaller number of tumors have been documented with cortisol levels that are not as high as expected after stimulation 35.
In light of the difficulty in obtaining CRH in our setting, we opted to use desmopressin stimulation in the diagnostic study, and the stimulation of the V2R receptor subtype, which these tumors express, allows ACTH to increase after its application 35,36. Also, it is more available in most centers in our setting.
Several studies have verified Oldfield's hypothesis of the laterality ratio, in which a value above 1.4 on the side with the highest concentration is suggestive of the side on which the hormone-producing tumor is found 37,38. This is important, as it guides the neurosurgeon towards the side to be resected in order to relieve the symptoms of hypercortisolism. In some studies, the sampling results have been better than imaging, with discordant results 39. Transsphenoidal surgery is currently considered to be the treatment of choice for Cushing's disease, and an initial cure is achieved in approximately 70-85% of cases; however, of the patients with initial remission, 10-15% will have a relapse months or years later. Radiosurgery is only considered as a second option in the event of failed surgery 40.
The lateralization of the lesion is highly relevant, since the ideal surgery is that which achieves complete removal of the tumor tissue and preserves the healthy pituitary. A broad surgical field is desirable when preoperative identification of the adenoma has not been obtained and the neurosurgeon must explore the whole gland in search of it. When it is not found, a hemi-hypophysectomy may be performed, choosing the side with the greatest ACTH secretion in the inferior petrosal sinus catheterization, if this was performed, or the side opposite the pituitary stalk when this is separated from the midline in the magnetic resonance (MR) images; or a total hypophysectomy, which practically guarantees a cure, but at the cost of panhypopituitarism 40.
Moreover, prolactin, a hormone produced in the anterior pituitary, is considered to be a biochemical marker which evaluates the success of petrosal sinus catheterization, as it is the only measurable hormone produced by the pituitary which is not altered by hypercortisolism, as occurs with growth hormone or thyroid stimulating hormone 41-43. Findling et al. showed that a sinus/peripheral ratio greater than 1.8 indicates a successful catheterization 44.
Despite being an invasive procedure, petrosal sinus venous sampling has a very low complication rate. The literature records up to 4% which are due to the puncture 45. Very rare cases of thrombosis, transient venous hypertension with pontine involvement, pulmonary embolism, transient sixth cranial nerve palsy, and subarachnoid hemorrhage have also been described 46. In our study, we chose to perform the procedure under general anesthesia as this facilitates the test and keeps the catheters from being shifted, for appropriate sampling. It should be highlighted that venous catheterizations are safer than arterial ones; we only used an arterial access in one case (3.7%), which minimizes the probability of complications. There were no immediate complications in our study.
Other alternative sampling sites include the jugular veins at the lowest petrosal sinus drainage point; however, the sensitivity decreases to 80%. Some authors use it first for diagnostic purposes, and only access the petrosal sinuses if there is a negative result 47-49. Bilateral sampling of the cavernous sinuses is also described 50, with higher ACTH concentrations compared to the petrosal sinuses 51. However, the ranges of sensitivity and specificity are similar to those of the petrosal sinuses; thus, it is not routinely used due to a greater risk of complications 52.
Conclusion
Bilateral petrosal sinus sampling is the best diagnostic test for central Cushing's syndrome. In our retrospective study, petrosal sinus catheterization was successful in a high percentage of patients with no immediate complications. We believe that the number of post-stimulation samples could be reduced to one. However, more studies are needed to validate this information before taking it as a recommendation. We consider bilateral petrosal sinus sampling to be a reliable diagnostic tool with a high technical success rate and few contraindications.











 text in
text in