Introduction
Trimethoprim-sulfamethoxazole (TMP-SMZ or cotrimoxazole) is an antimicrobial used to treat bacterial infections and as prophylaxis against opportunistic Pneumocystis jiroveci infections in immunocompromised patients. Currently, more than eight million prescriptions are written annually 1.
Its use at set doses in immunocompetent patients is safe, with a low (approximately 8%) rate of adverse events (AEs) 2. However, in the immunosuppressed population, the incidence of AEs can reach almost 83%. In our country, medication poisonings are in second place with 20.9%, for a total of 14,179 cases 3. Antibiotics are very important within this group, as they are frequently prescribed in clinical practice. Below, we present two cases of patients who intentionally took high doses of TMP-SMZ and were admitted to the emergency room, with their clinical picture and the medical treatment instated.
Case 1
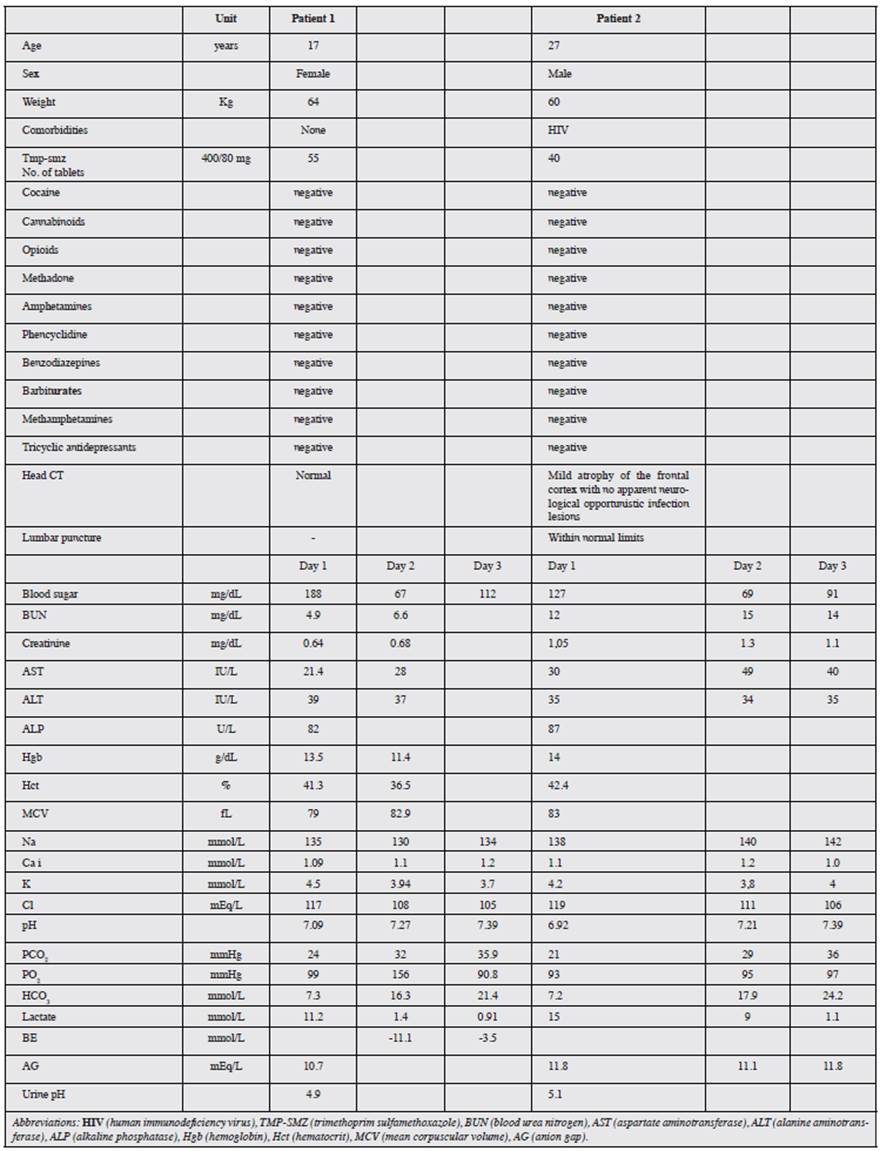
This case involved a 17-year-old woman with no significant medical history, who was admitted to the emergency room for self-ingestion of 55 TMP-SMZ tablets four hours prior, followed by emesis, stupor, a generalized tonic-clonic seizure and arterial hypotension. She subsequently experienced respiratory failure with the need for orotracheal intubation and admission to the Intensive Care Unit (ICU). Initial lab exams showed marked metabolic acidosis with hyperlactatemia (Table 1). Treatment was begun with general support measures and IV sodium bicarbonate. She later experienced moderate hyponatremic and hypoglycemic episodes. The patient progressed satisfactorily with successful extubation on the second hospital day. However, she had persistent metabolic acidosis which was only corrected on the fifth day, and she was discharged with outpatient follow up.
Case 2
This case involved a 27-year-old HIV+ man, with a history of opportunistic infections, who was admitted to the emergency room due to a clinical picture of emesis and drowsiness. Initial lab exams showed compensated metabolic acidosis with respiratory alkalosis and hyperlactatemia. He subsequently had three generalized tonic-clonic seizures with upgaze and without sphincter relaxation, which were managed with IV midazolam. These, together with episodes of psychomotor agitation, indicated the need to rule out neurological infection, and thus he underwent computerized tomography and lumbar puncture. He also had a urine test to rule out substance abuse, which was reported as negative, and follow up arterial gases showed persistent metabolic acidosis (Table 1). From the time of admission, he was treated with general support measures due to a suspected TMP-SMZ overdose, which was confirmed by the patient himself when he recovered consciousness, stating that he had taken a total of 40 tablets. His acid-base status was corrected on the fourth hospital day and he was discharged on the fifth day.
Discussion
Trimethoprim-sulfamethoxazole is a frequently prescribed antimicrobial worldwide. Although this medication is generally well tolerated, its use for over 40 years has allowed some AEs to be identified, both in adverse reactions and in overdose. Skin reactions have been seen, ranging from mild (such as rash) to severe (such as toxic epidermal necrolysis), with HIV patients being the most affected 1. When used for long periods of time, TMP-SMZ has been associated with blood disorders such as eosinophilia, anemia, thrombocytopenia, agranulocytosis and bone marrow aplasia 5. Cases of transient elevation of nitrogen-containing compounds or permanent kidney failure have been documented. It may also cause hyponatremia and hypercalcemia and, therefore, the concomitant use of angiotensin converting enzyme inhibitors and spironolactone should be avoided. Other, rarer, effects include liver toxicity, vertigo and pneumonitis 6.
In the clinical cases described above, we found metabolic acidosis with a normal or hyperchloremic anion gap, which excludes causes like lactic acidosis, toxins such as alcohols or salicylates, sepsis, kidney failure or respiratory failure. At the same time, one of our patients did not have a medical history while the other one, despite having a significant history of HIV, did not have current infections or gastrointestinal disorders which would explain bicarbonate wasting. Thus, the only remaining possibility was a renal cause such as renal tubular acidosis (RTA).
Lactic acidosis induction has been reported with high doses of parenteral forms which contain polyethylene glycol as a vehicle. However, this etiology is not possible in our patients as they ingested the oral form which lacks this carrier 7.
Although type 4 RTA is described in the literature as the most frequent TMP-SMZ-induced RTA, our patients did not have hypercalcemia, and therefore it was not considered. Furthermore, they had a urine pH below 5.5, which led us to conclude that they had a type 2 RTA, in which the proximal tubule is unable to reabsorb bicarbonate, unlike type 1 RTA where the distal tubule segments are unable to adequately acidify the urine, which leads to its alkalinization in the presence of metabolic acidosis 8.
Type 2 RTA tends to be seen in Fanconi tubular disorder, or secondary to the effect of some carbonic anhydrase inhibitors such as acetazolamide and topiramate. The clinical cases we reported did not have this history, but sulfamethoxazole has been reported to inhibit carbonic anhydrase, with the previously explained effects on bicarbonate 9.
There are no evidence-based or universally accepted treatment strategies for TMP-SMX-induced RTA. The literature reports that patients progress satisfactorily with withdrawal of the medication and general support measures which may, in some cases, include alkalinization of the medium with bicarbonate 10. Finally, the rapid resolution of the clinical picture in both cases with the therapeutic measures employed supports our hypothesis and the treatment instated.
The patient in the first case had modest hyponatremia which resolved on the third hospital day. When we compared this finding to the literature, we found that it is uncommon, but would be explained by the action of TMP on the distal tubule, since it is a weak heterocyclic base, with a structure similar to that of the potassium-sparing diuretics like amiloride and triamterene, which blocks sodium reabsorption in the epithelial sodium channel of the distal nephron 11. Hypercalcemia, a disorder which may be seen in 44-70% of those receiving TMP-SMZ, was not found in our patients. However, it is more likely to be reported in those with HIV who have needed long-term antibiotics either for treatment or prophylaxis 12, which was not true for our HIV patient, as he had not taken the medication in the six months prior to the overdose for which he consulted.
Finally, the hypoglycemia is caused by sulfamethoxazole which can increase insulin secretion from the pancreas, especially in overdose cases such as the ones we have reported together with kidney failure 13. This is probably due to the structural similarity between sulfamethoxazole and the sulfonylureas.
As we can see, although the AEs related to the use of TMP-SMX resolve after discontinuing treatment or lowering the dose, they are complications which should be identified promptly to decrease morbidity and hospital stay. Likewise, even more research is needed on the mechanisms of action involved, in order to prescribe appropriately, especially for susceptible individuals such as immunosuppressed patients (HIV-transplant and corticosteroid users).











 text in
text in