Introduction
Gastroesophageal reflux disease (GERD) occurs when retrograde flow of gastric contents causes uncomfortable symptoms and/or esophageal or extraesophageal compli cations 1. It affects 8-10% of the world's population and 11% of the Latin American population 2. In Colombia, it occurs in 12% 3. The diagnosis is clinically presumed when there is heartburn and/or regurgitation 4 and is conclusive when grade C or D esophagitis is found on endoscopy 5 or when the endoscopy is negative (70%) and impedance-pH monitoring is positive 5. The latter patients constitute what is known as non-erosive reflux disease (NERD) 5.
The cornerstone of treatment is inhibition of acid se cretion using proton pump inhibitors (PPIs), along with general measures such as losing weight if the body mass index (BMI) is greater than 25 kg/m2 (6,7, stopping smok ing 8, and controlling stress 9. Other lifestyle changes, although often recommended, lack high quality evidence to support them 10. Among the various first-generation PPIs, esomeprazole (ESO) causes greater gastric acid suppression and, like rabeprazole, is not metabolized by CYP2C19 in the liver 11,12. In Colombia, more than 75% of people are rapid or ultrarapid PPI metabolizers 13. In patients with GERD and esophageal erosions, a response is achieved in 85-90%, and when there is no esophagitis, in 55-60% 14,15. Only 54-68% of patients treated with PPIs adhere to treatment 16,17. Eighty-four percent of patients who respond to PPIs adhere to treatment compared with 50% of those who do not respond (p<0.0001) 18. When treatment is adhered to, the efficacy is 75% with one dose per day and 80-90% with two doses 19. Altogether, 75-90% of patients who do not respond to twice daily PPI dosing have an overlapping functional disorder such as hy persensitivity to reflux or functional heartburn (4, 20-22). Notwithstanding this knowledge, the experts and various treatment guidelines for this group of patients recom mend performing esophageal impedance monitoring with esophageal pH monitoring (impedance-pH monitoring) 23-27 and, more recently, providing low-dose visceral neuromodulators 28-30, especially tricyclic antidepres sants (amitriptyline, imipramine) 31,32, or selective serotonin reuptake inhibitors (SSRIs) like fluoxetine or citalopram 30,33,34.
Considering the high cost of impedance-pH monitoring and the fact that most patients who do not respond to two correctly prescribed doses of PPIs have an overlapping functional disorder, we decided to carry out this study to determine if optimizing GERD treatment (with a successive correction of incorrect prescriptions), adopting effective general measures, prescribing two doses of PPIs and, fi nally, adding visceral neuromodulators could cumulatively control the symptoms.
Materials and methods
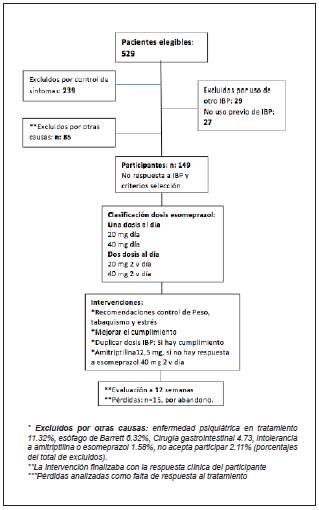
This was an open, prospective intervention study based on real data from daily clinical practice, carried out on a cohort of adult patients over the age of 18 with GERD seen as outpatients at the Centro de Gastroenterología y Endoscopia Digestiva de Bogotá, which is affiliated with the graduate gastroenterology program at the Universidad Nacional de Colombia. The inclusion criteria were: age 18 or older, typical heartburn and regurgitation symptoms, treatment with ESO, and lack of clinical response after 12 weeks of treatment. Patients who had GERD and were symptomatic despite treatment were referred to a special consultation with one of the authors (JL) who, in turn, discussed the cases with another one of the authors (WO). The exclusion criteria were pregnancy, lack of control of typical reflux symptoms, use of a PPI other than ESO, ESO or amitriptyline intolerance, treatment for a pyschi-atric disease (taking SSRIs or tricyclic antidepressants), having complications of GERD (peptic stenosis, Barrett's esophagus or esophageal cancer, as well as endoscopic findings suggestive of eosinophilic esophagitis [EoE]), prior upper gastrointestinal surgery, current or previous gastrointestinal cancer, esophageal motility disorders, and active debilitating comorbidities (coronary disease, cardiac arrhythmias, COPD, cirrhosis, chronic kidney disease, sclerosis, gastroparesis).
Interventions
During the initial visit, the patients' characteristics were identified and, as appropriate for each case, the ESO prescription was corrected and general recommendations were given: to lose weight, stop smoking and reduce stress. When there was adherence with no clinical response, the ESO dose was doubled, and the response was evaluated in the following 12 weeks. If at follow up there had been no response to treatment, optimization was once again performed, and a new assessment scheduled. Response to treatment was defined as the disappearance of symptoms or a decreased frequency to less than two times per week. Clinical response was determined through phone calls, appointments and medical chart review.
Statistical analysis
Quantitative variables are presented as summary statistics and measures of dispersion; qualitative variables are present ed in absolute numbers and proportions. McNemar's test was used to evaluate clinical response and adherence, Student's t-test was used to compare paired quantitative variables, and X2 was used for comparisons between qualitative variables, with p<0.05 considered statistically significant. A sensitivity analysis for losses was performed and these were defined as nonadherence or lack of clinical response to treatment. The SPSS-26 program was used for statistical analysis.
Results
A total of 529 eligible patients were found; 380 were excluded and 149 were ultimately selected (Figure 1).
General patient characteristics
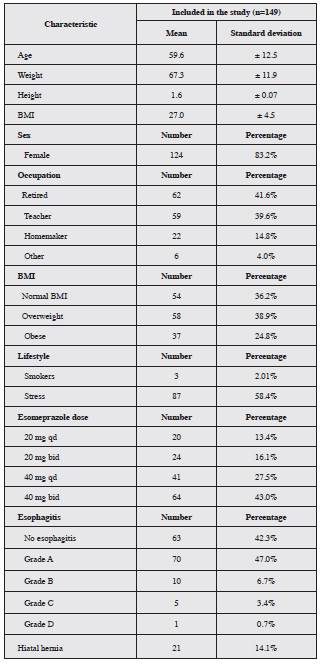
The average age of the study patients was 59.6 years ± 12.5. Most were in the 60-74 year age group (43.2%), fol lowed by the 35-59 year group (42.3%); women made up 83.2% of the population. The most common characteristics were overweight and obesity (38.9 and 24.8%, respec tively). The most frequent endoscopic finding was grade A esophagitis (47.0%); hiatal hernia was found in 14.1% of the participants. Table 1 summarizes the characteristics of study patients.
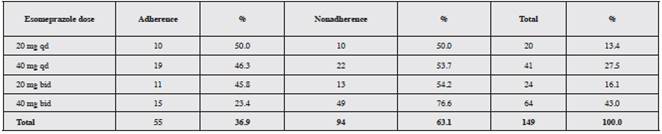
A total of 63.1% of participants did not take the treat ment appropriately; the ESO dose most associated with poor adherence was 40 mg twice a day (76.6%) (Table 2).
Study losses
The total losses amounted to 15 participants (10.1%), a lower percentage than expected (20%). The losses oc curred due to participant desertion and were analyzed as nonresponses or nonadherence to treatment.
General response
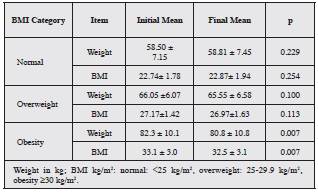
The assessment of the optimization intervention was carried out from August 2019 to March 2020. No relation ship was found between age and sex and clinical response to treatment or adherence. Weight loss in obese individuals is a key objective within the treatment optimization recom mendations for refractory reflux (Table 3).
Overweight patients achieved an average weight loss of 0.5 kg (95%CI -0.1-1.1) and a BMI change of 0.2 (95%CI -0.04-0.45). For obese individuals, the mean reduction in body weight was 1.43 kg (95%CI 0.36-2.51), with a mean BMI reduction of 0.6 (95%CI 0.17-1.04).
Clinical response to esomeprazole
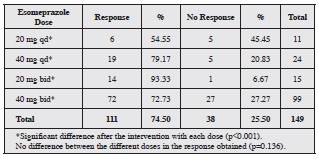
The clinical response to ESO of all the patients, even those who did not meet the criteria for using amitriptyline, is reported by dose in Table 4. Clearly, the clinical response improved significantly when treatment was optimized. The group receiving 20 mg twice a day had the highest percent age of clinical response (93.33%); the changes in the other groups are summarized in Table 4.
Clinical response to amitriptyline and the maximum dose of esomeprazole
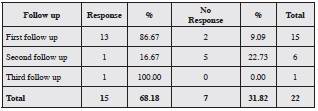
Twenty-seven patients did not respond to optimized treatment with the maximum dose of ESO. Of this group, five did not continue with the protocol, and the remaining 22 were treated with amitriptyline 12.5 mg each night, plus ESO 40 mg every 12 hours; of these, 15 had a clinical re sponse (68.18%; p<0.001). The most common adverse event was daytime sleepiness (eight patients, 53.3%; p=0.004) (Table 5).
With sequential treatment from PPI optimization to the addition of amitriptyline, a cumulative improvement in symptoms was achieved in 85% (95%CI 78.6-90.4) of the patients. At the end of the intervention, treatment adherence was found to have increased 44.3% (p<0.001). A direct relationship was found between adherence and response to treatment (with or without amitriptyline; p<0.001, respectively).
Discussion
The present study was performed in a cohort of patients drawn from routine outpatient care, where financial resources are lacking to exhaustively study patients with GERD. In addition, there is evidence that the lack of response to treatment in many patients includes an inadequate PPI prescription 35,36. Other causes include lack of adherence to treatment 16-18, insufficient acid inhibition with a single daily dose 19, the bioavailability of PPIs 11,12, nonacid gastroesophageal reflux 37, eosinophilic esophagitis 37, achalasia 37, obesity 7, smoking 8 and stress 9, or also an overlapping functional disorder or one of these disorders as the primary entity 20,21. Thus, the assessment of refractory patients should start with an adequate evaluation of symptoms and an esophagus with no endoscopic abnormalities or with erosive esophagitis (Los Angeles C or D) 5. In addition, the presence or absence of obesity, stress, and smoking should be determined and adherence to the prescribed PPI, etc. verified 7-9,16-19.
In this study, an intervention protocol was designed for patients classified as having refractory reflux due to an inadequate response to ESO treatment. The first step was treatment optimization, achieving symptom improvement in 75% of the patients previously classified as refractory, with similar results to those reported by Fass and Shapiro 15. Optimization has two fundamental pillars: the first is to ensure adherence, with which clinical response is achieved in 71.4% of cases. However, a percentage of patients do not improve, and the next step is to increase the PPI dose (twice a day), achieving improvement in 74.5% of patients. These results are more favorable than those reported by Fass and Murthy 38, who achieved symptom resolution in only 20% when they increased the dose to twice daily 39.
In the group of patients who did not respond despite the optimization strategies, we added a visceral neuromodulator (amitriptyline), in light of the fact that previous studies have proven that profound acid suppression is achieved when patients receive two correct doses of PPIs and, in 90%, symptom persistence is due to an overlapping func tional disorder 4. Charbel et al. 40 found that, when patients received two doses of omeprazole, only 3.8% had an abnormal esophageal pH. Another study found that the probability of normal pH monitoring using double PPI doses is 11 (95%CI 4.3-30.1 p<0.01) 40. In our study, 68.2% of the patients (15 out of 22) who received two doses of ESO showed clinical improvement when amitriptyline was added. Similar results were found by Faruqui et al. 32, who showed that when amitriptyline was added to pantoprazole, heartburn improved in 65% of the patients and regurgitation in 94.2%. No adverse effects were reported in that study, unlike our study in which 53% of the patients reported mild drowsiness that did not interfere with their daily activities. Recently, Abdallah et al. 20 found that when patients with and without a response to single-dose PPIs were compared, impedance and pH monitoring were similar in both groups with regard to the type of reflux (acid, weakly acidic and weakly alkaline), but 75% of the patients with no response to PPIs had an overlapping functional esophageal disorder. These results would justify the addition of a neuromodulator when symptoms persist despite the PPI. In our study, a second dose of PPI was added empirically, based on previous findings that when two doses of PPI are given, more than 96% of the patients achieve acid suppression 40. If there was no response, amitriptyline was added, considering the possibility of the coexistence of a functional disorder 4. The treatment of patients with refractory heartburn is com plicated and various approaches and strategies have been tried. This year, Vaezi et al. 42 reported that the use of a bile salt chelating agent, IW3718 (a special presentation of colesevelam which allows it to remain in the stomach and trap bile acids) at a dose of 1.5 grams twice a day, was able to decrease the heartburn score 11.9% more than the placebo at week eight (58 vs. 46%) (p=0.02). In that study, the included patients had to have had GERD symptoms refractory to single-dose PPI in the previous eight weeks 42. Those results could be explained by the noxious ef fect of conjugated bile acids on the esophageal epithelium 43-44; however, the advantage over placebo is only 12%. We believe further studies would be needed to determine if this novel form of colesevelam would be more effective than adding a second PPI dose for satisfactory acid suppression 19-22. Another empirical strategy for treating refractory heartburn is surgical treatment 45. Recently, laparoscopic Nissen fundoplication was compared with 20 mg of omepra-zole on an empty stomach and before dinner plus baclofen (a lower esophageal sphincter relaxation inhibitor), plus imipramine in patients with refractory heartburn 45. The surgical treatment was more effective than the medical treatment (67 vs. 28%, p=0.007); however, in each group, 40-50% of the patients had visceral hypersensitivity and the rest had abnormal acid reflux (impedance and pH monitor ing). This last finding shows that there was inadequate acid suppression and, therefore, duplication of the PPI dose would be indicated 19. If acid secretion was not correctly suppressed, it would be preferable to increase the PPI dose rather than performing surgery; PPI-refractory heartburn is not an indication for surgery 12. Given the fact that almost half of the patients had esophageal hypersensitivity, a placebo effect in the superiority of the surgery cannot be ruled out. Thus, we believe that the findings of this study cannot be extrapolated to other populations. In addition, the prevalence of rapid or ultrarapid PPI metabolizers in this population is unknown. With this information, the use of PPIs not affected by CYP 11,12 would be indicated if the prevalence of these genotypes is high. Several authors have emphasized that GERD treatment is not just suppressing acid secretion with higher doses of PPIs 4,32. The classical basic pathophysiological conception of GERD considers it to be secondary to an esophageal sphincter relaxation disorder which allows hydrochloric acid and bile to rise in the esophagus 46; however, GERD is much more complex and there are many individual phenotypes 46.
We believe that our study provides an empirical alterna tive for the routine treatment of patients with GERD, and these results add new evidence for not rushing to use imped ance and pH monitoring unnecessarily in patients who do not initially respond to PPIs, as has been previously suggested 23,26,27,41.
Our study has limitations. Esophageal biopsies were not taken in patients who continued to have symptoms de spite the use of a neuromodulator, to rule out eosinophilic esophagitis; there was a small sample; and we did not begin with first-time patients and follow them from single-dose PPI initiation onward, ascertaining the additional yield of each successive intervention to determine this approach's performance in real-life outpatient care in an underdeveloped country. However, we have found that treatment optimiza tion improves 85% of patients who do not respond to PPI treatment; therefore, we consider that treatment should be optimized using the strategy proposed in this paper before ordering esophageal impedance and pH monitoring.
Conclusion
This treatment approach for GERD is the first study in our country and in Latin America showing the benefit of PPI optimization in avoiding costly esophageal studies. The 85% cumulative success is very significant in the routine management of these patients. Further similar studies are needed with a greater number of patients to determine if this therapeutic approach would be an alternative for countries with financial constraints in health care.











 texto em
texto em