Introduction
Direct oral anticoagulants (DOACs) emerged in response to the search for an ideal anticoagulant, after warfarin had been the only available anticoagulant for a long time 1,2. The DOACs like rivaroxaban, apixaban, dabigatran, edoxaban and betrixaban (the first three of these available on the Colombian market), are widely recommended for their proven efficacy and greater safety when compared in patients with nonvalvular atrial fibrillation (AF) 3-6.
Warfarin is a medication directly related to hemorrhagic adverse events, with an estimated 3% frequency of major and fatal bleeding at three months 7, which could be higher in our setting due to lack of adherence to treatment, difficulties in patient education, irregular follow up and a low percentage of time in therapeutic range (TTR) 2,8. In contrast, DOACs have proven to have advantages over warfarin, like rapid onset of action, a predictable effect, low interindividual vari ability, few drug interactions, improved adherence, decreased hospitalization costs, and no need for periodic monitoring and frequent dose adjustments, along with decreased major bleeding as a fundamental safety outcome 9,10.
From a pharmacokinetics perspective, these medications are known to differ substantially in oral bioavailability, plasma protein binding and renal excretion 11,12. The latter is higher for dabigatran (80%), while with edoxaban, rivaroxaban, apixaban and betrixaban, 50, 33, 27 and 11% of the dose is excreted unchanged, respectively 3,13-15.
It is common to find patients with an indication for an ticoagulation in daily medical practice, mainly due to atrial fibrillation (AF), and many of these have chronic kidney dis ease (CKD) 16,17. Thus, conventional treatment must be studied to see if it is equally valid for this specific population.
The large, randomized trials which have evaluated the effectiveness and safety of direct anticoagulants vs. warfarin have, in general, excluded patients with advanced CKD and on dialysis, and therefore their treatment is currently controversial 18-21. So far, only observational studies with small sample sizes 22-24 and pharmacologically based studies are available as the sole support for making complex medical de cisions, but we still do not know if they are the best decisions for the patients and the healthcare system. Thus, the objective of this paper is to describe the sociodemographic, clinical and laboratory characteristics of anticoagulated individuals with advanced CKD in stages 3, 4 and 5 at IPS Universitaria Universidad de Antioquia in the city of Medellín (Colombia), and to report the cases of minor, major and fatal hemorrhage which occurred.
Materials and methods
Study design
This is a descriptive, retrospective cohort study on the safety of anticoagulants in patients with stage 3, 4 and 5 CKD.
Location
From January 2017 to December 2018, the medical charts of anticoagulated patients seen in the outpatient program of the institution's "anticoagulation clinic" were followed.
Participants
The inclusion criteria were: patients over the age of 18 with a prior diagnosis of CKD made by the attending physi cian or with creatinine clearance < 60 mL/min/1.73 m2 on two consecutive measurements at least three months apart, and who required anticoagulation for at least three months.
Patients without a clear diagnosis or without a known indication for anticoagulation, those who only had an abnor mal creatinine level on follow up, and patients who were not able to be seen a minimum of two times at least three months apart, were excluded.
Variables
The analyses included variables such as: sex; age; type of health insurance; indication for anticoagulation; anticoagulant and, if warfarin, INR at each visit and when bleeding, as well as TTR. The CKD stage, comorbidities and medications taken prior to anticoagulation were also included, along with major or minor bleeding, the affected organ, need for transfusion, GFR and comorbidities. Functional class was determined according to the medical chart report.
Chronic kidney disease is defined as the presence of ab normal kidney structure or function for at least three months, with health repercussions. Any of the following markers of kidney damage should be present for three months: elevated albuminuria, urinary sediment abnormalities, electrolyte abnor malities or other tubular dysfunctions, histological structural abnormalities, structural abnormalities on imaging tests, kidney transplantation or evidence of GFR < 60 mL/min/1.73 m2 (25. (The more than three-month duration of some of these abnor malities was determined based on the medical chart.)
The kidney disease stage was assessed through serum creatinine calculation using the CKD Epidemiology Collaboration (CKD-EPI) formula, as recommended by the 2012 KDIGO guidelines (25) and based on GFR, as fol lows: normal (G1) GFR > 90 mL/min/1.73 m2, G2: 60-80 mL/min/1.73 m2, G3a: 45-59 mL/min/1.73 m2, G3b: 30-44 mL/min/1.73 m2, G4: 15-29 mL/min/1.73 m2 and G5: <15 mL/min/1.73 m2.
The International Society on Thrombosis and Haemostasis (ISTH) criteria were used to assess bleeding 26.
Major bleeding: clinically evident bleeding which meets one of the following criteria:
Hemorrhage resulting in a fall in hemoglobin level of 2 g/dL or more for a 24-hour period.
Bleeding which requires the transfusion of two or more units of packed red blood cells.
Bleeding at a critical site (intracranial, intraspinal, intraocular, pericardial, intraarticular, intramuscular with compartment syndrome or retroperitoneal).
Bleeding leading to death.
Minor bleeding: a clinically evident hemorrhagic event which does not meet any of the previous criteria for major bleeding but leads to:
Hospital admission for hemorrhage.
Physician-guided medical or surgical treatment for bleeding.
A change in antithrombotic therapy.
Fatal bleeding: defined as a hemorrhagic event that is the main cause of death or directly contributes to death.
Source of the data
The anticoagulation clinic database and medical charts of IPS Universitaria Universidad de Antioquia.
Biases
Due to the descriptive nature of the study, there are biases related to the lack of patient randomization. The population's lack of representativeness is also implicit, along with some missing data in the medical charts. To control the biases, the medical charts of different specialists were evaluated on random dates and during appointments at the anticoagula tion clinic.
Sample size
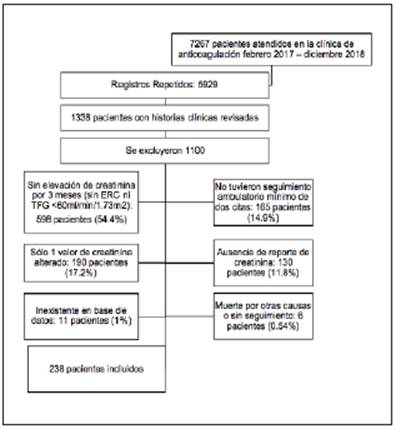
Since this was a descriptive study, sample size was not calculated and, for convenience, all patients seen during the study period were selected. Out of 1,338 patients seen in the anticoagulation clinic, 238 medical charts which met the mentioned inclusion criteria were analyzed.
Statistical methods
Nominal variables are presented as absolute and relative frequencies. Continuous variables are presented using aver ages and standard deviation. The only missing value in the medical chart data was race, which was assumed to be "not African American" for all patients, in order to apply the CKD-EPI formula to obtain the GFR.
The Rosendaal method was used to calculate TTR.
The database was created using the Excel 2017 (Micro soft Corp.) program, and IBM SPSS Statistics 20 statistical software was used for analysis.
The project was presented to IPS Universitaria Universidad de Antioquia and was studied and approved by this institu tion's Ethics and Research Committee. Bioethical principles were taken into account in the conduction of this study; results were obtained without violating patients' rights and with the objective of improving understanding of the diseases for the benefit of the affected population and medical research. As it was a descriptive study, informed consent was not obtained from the patients.
Results
A total of 7,267 care encounters were recorded in the anticoagulation clinic's database between January 2017 and December 2018. After removing duplicate records, 1,338 medical charts were reviewed to select those which met the inclusion criteria, for a total of 238 patients (Figure 1).
The main reason for anticoagulation was AF (81.9%), followed by venous thromboembolic disease both in lower limbs and the lungs (7.6%). The most frequent age group was 81-90 years (39.1%), the sex distribution was similar with 52.1% women and 47.9% men, and 65.5% had contributive and 34.5% had subsidized health insurance.
The most frequently used anticoagulant was warfarin (45%), followed by rivaroxaban (31.5%) and apixaban (14.3%). A total of 36 patients changed anticoagulant during follow up (Table 1).
The average TTR in patients on warfarin was 48.8% and the median was 51% (0-100% for minimum and maximum values, respectively).
The majority of patients were in stage G3a (37.3%) and G3b (40.7%) CKD, and the greatest frequency of bleeding was in this stage. There were only 5.8% in G5 and, of these, 100% were on renal replacement therapy (Table 2).
Out of all the patients studied, bleeding was seen in 20 (8.4%), and of these, 7 (35%) had major bleeding. Warfarin was the anticoagulant associated with 50% of these cases, followed by rivaroxaban (20%) and apixaban and dabigatran (15% each). The most frequent site of major bleeding was the digestive tract (five patients); there was only one case of fatal bleeding in a 95-year-old patient with stage 3b kidney disease who was receiving apixaban and had a seizure, mod erate head trauma and central nervous system (CNS) bleed ing. The remaining hemorrhages were minor (13 patients); 46.1% were due to warfarin and 15% to dabigatran. The most common site for these bleeds was soft tissues (30%).
Of the 10 patients who bled on warfarin, six had subthera peutic INRs, three had an INR in the 2-3 range and one had an INR less than 2.0 (Table 3).
The description of each of the subjects who bled is shown in Table 4.
Discussion
This study described stage 3, 4 and 5 CKD patients who were seen at the anticoagulation clinic at IPS Universitaria Universidad de Antioquia in Medellín, Colombia over a period of two years.
It is common to find patients with an indication for antico agulation in daily clinical practice, due to the high prevalence of AF which increases with age, being approximately 2.3% at age 40, and greater than 6% in those over 65 27. Fur thermore, CKD, with a prevalence of approximately 12% in the general population (2 per 100 population in Colombia) in 2014 28, has been defined as an independent cardiovascular risk factor as it has been pathophysiologically related to both thrombotic and hemorrhagic events 29,30.
Approximately one out of every five patients with CKD has AF, which is 2-3 times more than in the population without CKD 31. For those on peritoneal dialysis, the prevalence is up to 7%, and for hemodialysis, it is 13%. The percentage of patients with AF and CKD also increases with age 32. Our study showed that 51.7% of the patients were over the age of 70.
According to the literature, AF is the most common rea son for anticoagulation in patients with CKD. In our study it accounted for 81.5% of the patients, similar to the findings of international reports 33,34.
With regard to the safety of anticoagulants, a study per formed in Canada with 1,626 patients on hemodialysis who took warfarin yielded an adjusted HR for bleeding of 1.44 (95%CI: 1.13-1.85), and the authors suggest that the risk-benefit profile does not support the routine use of warfarin to reduce cerebrovascular accidents in patients on hemodialysis 35. A meta-analysis in 2007 found that those with terminal CKD who were treated with warfarin had a 10 times greater risk of major bleeding than the general population and that these events occurred even when the INR was between 1.5 - 2.0 36. In our study, out of 107 patients on warfarin, 10 bled (9.34%); of these, only two were using ASA and not all were over anticoagulated at the time of bleeding. There was a 5.6% rate of minor bleeding and 3.7% rate of major bleeding, which is lower than that of Kooiman et al.'s (2014) study which reported 15.6% for the latter type of bleeding, keeping in mind that the sample in this study was larger (n=724) 37. This study also reported bleeding in patients with INRs within the therapeutic range, which was found in our study (two patients with major bleeding and one with minor bleeding).
Despite the greater proven safety of DOACs, in our study we found that warfarin was the most commonly used medication (45.0%), even though 65.5% of the patients had contributive health insurance. This could lead us to question the reason for choosing warfarin over the rest of the antico agulants and to consider whether the weight of evidence in the Colombian population is sufficient for choosing the type of anticoagulant, or if social, economic and cultural factors have a greater weight when prescribing them.
An important outcome for patients on warfarin is TTR, which estimates the percentage of time during which the INR is within the desired treatment range, and is widely used as an indicator of anticoagulation monitoring 38. This study found a TTR of 48.8%, which means that for more than half the time, the patients were outside the target. This target failure is well described in the literature, such as by Chaaban et al. (2015) who presented data on the use of warfarin in patients on hemodialysis (HD) versus those with normal kidney function and found that not only did HD patients have more hemorrhages, but the INR was only within the therapeutic range 25% of the time in this group 39. Yang et al. (2017) recently published their study in which they found that only 21% of patients on dialysis had a TTR greater than 60%; in addition, they found that for 30% of the time, the INR was notably outside the range (INR less than 1.5 or greater than 3.5) 40. All of this makes antico agulation with warfarin entirely unpredictable and creates the possibility of carrying out more studies on this topic, given that the population is different, possibly older, with serious associated illnesses and difficulties in accessing the healthcare system and adhering to treatment.
With regard to the DOACs, apixaban is approved at a dose of 5 mg twice a day, which needs to be adjusted to 2.5 mg every 12 hours for patients who meet two of the following criteria: creatinine greater than 1.5 mg/dL, over the age of 80, or weight under 60 kg, according to the ARISTOTLE study 41. This study excluded patients with a GFR less than 25 mL/min, and thus the population with stage 4 and 5 CKD is not well represented. In spite of this, the updated 2019 AHA guidelines for AF and the FDA recommend using apixaban for patients with advanced CKD, including those on hemo-dialysis 42. This continues to be controversial because the recommendation is based on a pharmacodynamics study with 16 patients and a US Medicare register, and therefore the quality of the scientific evidence is not the best 43,44. In 2019, preliminary results of the RENAL-AF study were presented, comparing apixaban vs. warfarin on dialysis. The study was not conclusive, as it did not achieve the expected enrollment and thus was stopped before the stipulated time. In addition, the TTR for patients on warfarin was 44%, with a large proportion of patients in the subtherapeutic range 45. Our study showed that out of 34 patients on apixaban, three had bleeding (a rate of 8.8%). One of these was the only case of fatal bleeding; however, it was associated with head trauma which was the cause of death, this patient was elderly, and he had a GFR of 28 mL/min and therefore had an adjusted medication dose.
Likewise, rivaroxaban, in its main study (ROCKET-FA), excluded GFR < 30 mL/min 46. There are observational and pharmacological studies which demonstrate the safety of rivaroxaban in stage G4 CKD, and therefore it is approved in the United States for this indication, while other countries like Canada only use its traditional recommendation 47,48. In our study, 4 out of 75 subjects on rivaroxaban had bleeding (a 5.3% rate of bleeding) and all of these were in stage 3 CKD, except for one who required temporary renal replacement therapy.
Dabigatran is contraindicated for patients with GFR < 15 mL/min, according to international guidelines, but are there are no studies with large numbers of patients even for stage 4 CKD. Our study shows that this medication had the highest rate of bleeding (37.5%). Therefore, taking into ac count its high rate of renal excretion, we consider that it is not the drug of choice in patients with CKD 49. Even so, it is approved by the FDA for an adjusted dose of 75 mg every 12 hours based on a pharmacological prediction model 50.
The efficacy of oral anticoagulants has been very well established in controlled studies and more information is needed on their safety in different populations. This study described the clinical profiles and hemorrhagic events of anticoagulated individuals during follow up by IPS Uni versitaria Universidad de Antioquia from January 2017 to December 2018. This study represents the largest cohort published to date of patients with chronic kidney disease and the use of anticoagulants (both warfarin and DOACs) in Colombia and Latin America.
The limitations of the current study are related to its ret rospective nature, data collection and the absence of some data. Calculating the glomerular filtration rate using the CKD-EPI equation has some limitations given that race was not recorded for the vast majority of patients. The popula tion in stage G5 CKD was scant. Neither CHADs2Vasc nor HAS-BLED were considered for interpreting the results for patients with AF.
Conclusion
The most common reason for anticoagulation was AF and the most frequent kidney disease stage was G3. Over all, there was a low rate of major bleeding, which could be related to close follow up at an anticoagulation clinic. This type of bleeding was more frequent in patients on warfarin, which was associated with a low TTR (48.8%). The safest medications were apixaban and rivaroxaban with adjusted doses.
The number of patients in stage G5 CKD was very low, and therefore conclusions cannot be drawn regarding this particular group.
Randomized clinical trials are needed to determine the safest anticoagulation strategy in this population.











 texto en
texto en