Introduction
Therapeutic apheresis is an extracorporeal procedure in which blood removed from a patient is separated into its components, allowing only the desired elements to then be returned to the patient 1, although today, using special cascade techniques and added filters with absorption fibers, specific plasma elements thought to be mediators in disease processes can be removed 2,3. Plasmapheresis refers to an apheresis procedure in which plasma is extracted but not replaced, while therapeutic plasma exchange (TPE) is a technique in which the blood passes through a device which separates and removes plasma from the cellular components. This extracted plasma made up of monoclonal paraproteins or autoantibodies, is discarded and replaced with a colloid or crystalloid solution 4. In our institution, we have the availability of hydroxyethyl starch (HES), Voluven, which we use as a replacement solution in plasmapheresis therapy as an alternative to plasma and albumin, for both renal and non-renal indications, as long as the patient is not hemodynamically unstable and does not have an acute kidney injury. This is based on the recommendation that HES should be avoided in critically ill patients, due to its association with acute kidney injury and coagulation disorders. Voluven is smaller than other HES products, and various reports have associated it with fewer coagulation disorders and less impact on renal function. Some authors have shown different results, with even improved renal function and intravascular volume in hypovolemic patients 5.
The technique used at Hospital de San José, in Bogotá, is based on an extracorporeal circulation system, the Multifiltrate system (Fresenius Medical Care), using the PlasmaFlux® P1dry/P2dry plasma filter with pores that allow various protein components to pass, such as immunoglobulins, lipoproteins and other molecules with greater molecular weight, even those over two million daltons 6. No anticoagulation is used during plasmapheresis sessions.
Furthermore, plasma apheresis, which is widely used, has renal and non-renal indications, with different levels of evidence described by the American Society for Apheresis, and is a long-standing and well used treatment. There are few studies in the literature on its mechanism of action, but especially on its clinical and paraclinical response. Thus, the purpose of this study is to describe the experience in our institution, specifying the indications for plasma apheresis in the treated patients, indicating the technique utilized, and evaluating adverse events with regard to possible associated hematological or fluid and electrolyte disorders or kidney dysfunction. We seek to contribute to national and international research to support the various levels of evidence in the indications and safety of our technique.
Methods
An observational, descriptive, case series study was carried out. All available patients in the database of the nephrology department at Hospital de San José who met the eligibility criteria: patients over the age of 14 who were treated with therapeutic apheresis between January 2012 and April 2019, were included. Patients with incomplete medical charts were excluded.
Recruitment strategies
Billing records from the renal unit at Hospital de San José in Bogotá for plasmapheresis sessions from January 2012 to April 2019 were selected. Subsequently, the medical charts were requested from the medical records department at Hospital de San José in Bogotá, and the paraclinical exams of the selected patients were requested from the laboratory coordinator. Information was obtained from the medical charts, such as the diagnosis indicating the need for therapeutic apheresis; the number of sessions; serum calcium levels-coagulation times-blood urea nitrogen-creatinine before and after each therapeutic apheresis session; the order for therapeutic apheresis; adverse events related to plasmapheresis, specifically related to bleeding or symptoms of hypocalcemia; and clinical response to therapeutic apheresis. The data were collected by one of the investigators, stored on a Microsoft Excel sheet, and analyzed using STATA. 13e.
Analysis plan
The qualitative variables were reported using absolute and relative frequencies. Quantitative variables were reported using measures of central tendency and dispersion (median and interquartile range [IQR]).
Ethical considerations
The data were obtained confidentially and processed by the principal investigator, as described in Law 1581 of 2012, Article 2, Section (a) of the general law for the protection of personal information in Colombia. Likewise, following Resolution 8430 of 1993 of the Colombian Ministry of Health, the study is considered to be no-risk, since it uses retrospective document research methods and techniques and does not carry out any intentional intervention or modification of biological, physiological, psychological or social variables of the participating individuals.
The study protocol was approved by the Research Committee of the Fundación Universitaria de Ciencias de la Salud and by the Medical Ethics Committee, and did not require written informed consent.
Results
A total of 96 patients were included who met the inclusion criteria and none of the exclusion criteria. The median age was 35.5 years (IQR: 27-54). The minimum age was 16 years, and the maximum was 79 years; 61.5% were women and 38.5% were men. The median number of sessions per patient was 6 (IQR 5-7). The most common diagnosis was acute humoral rejection in kidney transplantation (30.2%), followed by alveolar hemorrhage secondary to SLE in patients refractory to first line treatment or with severe, life-threatening illness, and renal replacement therapy-dependent small vessel vasculitis (20.8%). By disease groups, the most frequent were neurological diseases, at 38% (Table 1).
Table 1 Diagnoses of patients treated with therapeutic apheresis.
| Diagnoses | ASFA2019 Category | Patients | Sessions | Sessions per patient | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Neurological diseases | ||||||
| Optic neuromyelitis | II | 15 | 15.6 | 85 | 14.0 | 6 |
| Myasthenic crisis | I | 9 | 9.4 | 54 | 0.9 | 6 |
| Autoimmune encephalitis | II | 10 | 10.4 | 65 | 10.7 | 7 |
| Multiple sclerosis | II | 1 | 1.0 | 10 | 1.6 | 10 |
| Hematological diseases | ||||||
| TMA | I | 6 | 6.3 | 44 | 7.2 | 7 |
| Hyperviscosity syndrome | I | 2 | 2.1 | 11 | 1.8 | 6 |
| Hemolytic-uremic syndrome | I | 1 | 1.0 | 5 | 0.8 | 5 |
| Bone marrow infiltration syndrome | II | 1 | 1.0 | 5 | 0.8 | 5 |
| Renal diseases | ||||||
| Humoral rejection in renal transplantation | I | 29 | 30.2 | 172 | 28.3 | 6 |
| Rapidly progressive glomerulonephritis | I | 1 | 1.0 | 5 | 0.8 | 5 |
| Rheumatological diseases | ||||||
| Alveolar hemorrhage (SLE, vasculitis) | II | 20 | 20.8 | 147 | 24.2 | 7 |
| Others | ||||||
| Ocular cicatricial pemphigoid | II | 1 | 1.0 | 5 | 0.8 | 5 |
| Total | 96 | 1 | 608 | 74 | ||
| TMA: thrombotic microangiopathy. | ||||||
The most frequently used replacement solutions were plasma + 0.9% NSS at a 50%/50% ratio and HES + 0.9% NSS 70%/30%, at 49.3% and 20.4%, respectively. (Table 2).
Table 2 Type of replacement solution.
| Type of replacement solution reemplazo | n | Percentage (%) |
|---|---|---|
| FFP - 0.9% NSS 50%/50% | 300 | 49.3 |
| HES - 0.9% NSS 70%/30% | 124 | 20.4 |
| FFP 100% | 83 | 13.7 |
| HES - SSN 0.9% 50%50% | 36 | 5.9 |
| FFP - 0.9% NSS 70%/30% | 29 | 4.8 |
| FFP - 0.9% NSS 60%/40% | 10 | 1.6 |
| FFP - 0.9% NSS 80%/20% | 8 | 1.3 |
| HES - 0.9% NSS 60%40% | 7 | 1.2 |
| Albumin 0.9 % NSS 50%/50% | 4 | 0.7 |
| FFP - Albumin 50%/50% | 4 | 0.7 |
| HES - 0.9% NSS 80%20% | 3 | 0.5 |
| FFP: fresh frozen plasma. NSS: 0.9% normal saline solution. HES: hydroxyethyl starch. |
Clinical variables by replacement fluid used
Table 3 shows the different clinical variables adjusted by subgroups according to the replacement fluid used in therapeutic apheresis, evaluated before and after therapy, and the subgroup of patients who also required renal replacement therapy (RRT).
Table 3 Lab results before and after therapeutic apheresis.
| Values before therapeutic apheresis | Values after therapeutic apheresis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Without RRT | With RRT | Without RRT | With RRT | ||||||
| Laboratory test | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | |
| PLASMA | Calcium | 7.9 (7.3 - 8.4) | 31 | 6.7 (6.3- 7.9) | 9 | 7.7(7.1-8) | 30 | 6(7-7.9) | 8 |
| Albumin | 2.4 (2 - 3.5) | 31 | 1.9(1.3 - 2.5) | 11 | 2.4(2-3.5) | 31 | 2(1.4-3) | 12 | |
| Hemoglobin | 10.7(8.1 - 13.1) | 54 | 7.4 (6.7 - 8.9) | 15 | 10.9(8.1-12.8) | 49 | 8.8(7.3-9.3) | 13 | |
| Creatinine | 1.4(0.7 - 2.5) | 55 | 3.7(2.5 - 5.2) | 14 | 1.3(0.65-2.2) | 52 | 2.1(1.6-4.7) | 12 | |
| GFR MDRD | 55 (27 - 94) | 55 | 59.5(31.5-122) | 52 | |||||
| PT | 12.2 (11.1 - 12.9) | 45 | 12.1(11.1 - 13.8) | 12 | 11.3(10.7-12.6) | 45 | 11.3(10.8-12.4) | 13 | |
| PTT | 31.8 (27.1 - 41.9) | 45 | 35.3(28.7-57.5) | 12 | 30.3(26.9-55.2) | 45 | 37.8(31.7-53.6) | 13 | |
| Platelets | 152,500 (112,000219,000) | 54 | 133,000 (76,000203,000) | 15 | 124,000 (87,000159,000) | 49 | 93,000 (85,000149,000) | 13 | |
| Corrected calcium | 8.9(8.4 - 9.5) | 20 | 8.2(8-9) | 9 | 8.9(7.8-9.6) | 20 | 7.6(6.4-9.3) | 7 | |
| HES | Calcium | 8.1(7.4-8.5) | 18 | 7.1(6.2-7.4) | 20 | ||||
| Albumin | 2.4(1.9-3.1) | 23 | 2.4(1.9-3.1) | 24 | |||||
| Hemoglobin | 13.4(10.5-15.6) | 24 | 12(10.1-14.7) | 25 | |||||
| Creatinine | 0.7(0.6-0.9) | 25 | 0.8(0-6-0.9) | 25 | |||||
| GFR MDRD | 106(88-145) | 25 | 104(78-147) | 25 | |||||
| PT | 12.3(11.7-14.5) | 21 | 12.1(10.8-14.4) | 23 | |||||
| PTT | 30.3(27.9-39.1) | 21 | 30.6(27.4-47.3) | 23 | |||||
| Platelets | 234,000 (186,000276,000) | 23 | 124,000 (87,000171,000) | 25 | |||||
| Corrected calcium | 9.1(8.6-9.5) | 16 | 7.9(7.1-9) | 18 | |||||
| RRT: renal replacement therapy; GFR MDRD: glomerular filtration rate (186.3*Serum creatinineA(-1.154)*AgeA(-0.203)*0.762*(if female)*1.18(if African American); IQR: interquartile range; HES: hydroxyethyl starch. | |||||||||
Hydroxyethyl starch replacement was not used in any patients who underwent RRT. An important finding in this subgroup of patients was a decreased platelet count after finishing therapeutic apheresis treatment, with an initial level of 133,000 (IQR 76,000-203,000) and later level of 93,000 (IQR 85,000-149,000). The other analyzed variables like calcium, albumin, hemoglobin and coagulation times were no different in this group before and after therapeutic apheresis. The group who underwent HES without RRT had a median platelet count before and after therapy of 234,000 (IQR: 186,000-276,000) and 124,000 (IQR: 87,000-171,000), respectively.
Patients who were treated with HES were found to have a median prothrombin time (PT) before therapeutic apheresis of 12.3 seconds (IQR: 11.7-12.9) and a median partial thromboplastin time (PTT) of 30.3 seconds (IQR: 27.9-39.1). After apheresis, the results were a median PT of 12.1 seconds (10.8-14.4) and a median PTT of 30.6 seconds (27.4-47.3), showing that the results remained within the normal range, which was similar in patients treated with plasma (Table 3, Figure 1).
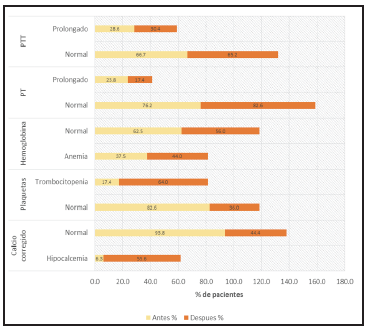
Figure 1 Changes in PTT, PT, hemoglobin, platelets and calcium before and after plasmapheresis, replacement with Voluven (HES).
In addition, patients in the HES group had a normal corrected calcium percentage before apheresis at 93.8 and 44% after apheresis. However, in patients whose corrected calcium went from a normal value to hypocalcemia, this change was not severe, as documented by a review of the medical charts in which there was no report of adverse effects secondary to this change, and this was similar to the plasma results as shown in Figure 3.
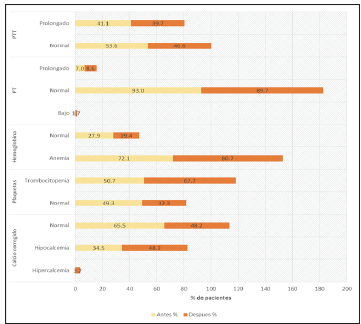
Figure 2 Changes in PTT, PT, hemoglobin, platelets and calcium before and after plasma-pheresis, replacement with plasma.
For formula-estimated glomerular filtration rates (eGFR) as calculated by the Modification of Diet in Renal Disease Study (MDRD-4) mL/min/1.73 m2 in the HES and plasma subgroups of patients not undergoing RRT, renal function was found to be preserved in the HES group, with 106 mL/ min (IQR 88-145) before and 104 mL/min (IQR 78-147) after apheresis. In the plasma group, the eGFR by MDRD was 55 mL/min (IQR: 27-94) before and 59.5 mL/min (IQR:31.5-122) after (Figure 3).
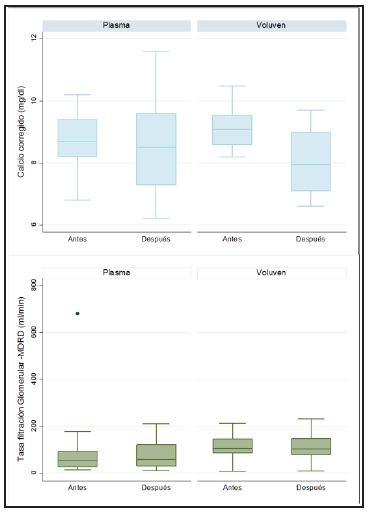
Figure 3 Glomerular filtration rate and corrected calcium before and after plasmapheresis, according to the type of replacement.
No adverse effects related to bleeding or hypocalcemia secondary to apheresis therapy were recorded in the medical charts reviewed. The doses of plasma volume exchange therapy were mostly 1-1.5.
Discussion
Historically, therapeutic apheresis has been performed more often for neurological and hematological disorders. Today, most of the procedures are performed for the same reasons; however, there has been an important change in its trajectory, with an increase in the number of plasma exchange procedures due to renal diseases, mostly Category I according to the American Society for Apheresis. In our experience, the most common diagnosis was acute humoral rejection in kidney transplantation, accounting for 30.2% of the cases.
At Hospital de San José in Bogotá, Colombia, 608 plasmapheresis sessions were performed during the study period, with different neurological, renal, rheumatological and hematological indications in 37.6%, 31.2%, 20.8% and 10.4%, respectively.
Fresh frozen plasma is the most frequently used replacement fluid 1, which concurs with this study. In our department, the preferred replacement fluids for plasma exchange in renal and non-renal indications, in patients with no prior renal function disorders, were a combination of 0.9% saline solution plus HES in 30/70%, 50/50% or 20/80% concentrations, respectively, and we found no serum creatinine abnormalities before and after each session. In fact, even after finishing the cycle of therapeutic apheresis sessions, no patients had an altered GFR. Our results are similar to those of two studies reported in the literature on the use of HES. The first study was by Muthuchellappan et al. 10 who, over a period of five sessions per patient, used HES as the replacement fluid plus two units of plasma after every session in order to avoid complications related to coagulation disorders, finding improved levels of creatinine and blood urea nitrogen (BUN) after the plasma exchange sessions. Another study by Pagano et al. at Washington University 11, in which they performed one session of leukapheresis using HES as the replacement fluid, reported stable renal function after therapy.
The nephrology department at Hospital de San José in Bogotá, Colombia, presented a study in 2017 analyzing the characteristics and clinical outcomes of patients with one or more episodes of acute humoral rejection who received therapeutic apheresis treatment and adjuvant therapies (with thymoglobulin, gammaglobulin and rituximab in 55.56%, 29.63% and 18.52% of the study cases, respectively). Altogether, 70.37% of the therapeutic apheresis sessions were performed with HES, and 29.63% with saline solution and albumin or plasma (when abnormal coagulation tests were found), finding stable kidney function based on the glomerular filtration rate calculated by the MDRD-4 formula, describing a GFR increase of more than 10 mL/min/year in 23% of cases, between 5-10 mL/min in 38.5% and less than 5 mL/min in 73.08% of the patients 12.
To carry out therapeutic apheresis by filtration, the literature describes the use of anticoagulants: calcium citrate and heparin; in our technique, these are not used in either continuous or intermittent infusion during apheresis therapy. We know that these anticoagulants can favor hypocalcemia or coagulation disorders. Although a plasma volume exchange eliminates approximately 63-75% of the plasma proteins, including the coagulation factors, most of the coagulation factors require 15-20% of their activity levels for normal hemostasis, and their levels are recovered within two to three days after ending plasmapheresis 13. We found similar data in our results measured by PT and PTT, and there were no recorded cases of bleeding related to the technique. In our sample, no patients had serum fibrinogen measurements, another lab test which reflects hemostasis disorders. A limitation of our study is not having pre and post-treatment measurements of fibrinogen levels, which is especially important when plasma is not used as the replacement fluid. However, we found no significant changes in coagulation times nor in platelet counts with either plasma or HES.
In our results, calcium levels decreased at the end of treatment, both with HES as well as plasma, with no symptoms or electrocardiographic abnormalities associated with hypocalcemia documented in the charts. However, it must be recognized that calcium was not measured at all sessions, nor were its corrections documented, with this being a limitation of our study, as we did not consistently measure serum calcium levels before each plasmapheresis session. Therefore, we recommend that management guidelines must be created so that the different areas within the institution ensure a safe and effective therapeutic exchange 13-14.
With these results, we seek to contribute a broad, complete and useful record to the clinical research literature in our country, this being the largest register of therapeutic apheresis, with similar results to those in the international literature, and our experience in preserving renal function with the use of HES.











 text in
text in 


