Introduction
Spiders are arthropods which are widely distributed throughout the world. More than 35,000 species have been described, however fewer than 1% are of clinical interest due to the lethality of their venom 1,2. In Colombia, arachnidism is third (11%) after ophidism (47.5%) and scorpionism (25%) in terms of the number of reports in most of the national territory, mainly in the departments of Antioquia, Valle del Cauca, Cundinamarca and Santander 3,4. Cases involve four main genera: Theraphosa (tarantulas), Latrodectus (black widow), Phoneutria (banana spiders) and Loxosceles (recluse, violin or brown spider) 5, with loxoscelism being the most epidemiologically important arachnid accident due to its broad geographic distribution and potent venom, capable of causing intense cutaneous necrosis and, in some cases, severe organic involvement.
The aim of this paper is to highlight the correlation between clinical signs and symptoms and the toxinological characteristics of arachnid venom, presenting the first case report of arachnidism in the context of HIV infection and an updated review of the literature, in order to motivate the readers regarding this forgotten tropical disease which may present as a real challenge in clinical practice. The patient provided informed consent for the information described below.
Case report
A previously healthy 48-year-old man with risky sexual behavior was admitted to the emergency room with a complaint of an animal having traversed his right leg while he was sleeping, with subsequent pain and intense itching on the dorsum of the ipsilateral foot, and the onset of an erythematous macular rash. He then reported that he found a brown, approximately 3.0 cm diameter spider with thin legs in his bed, with no other details given. Over the following hours, he developed asthenia, adynamia, headache and diffuse muscular and osteoarticular pain, leading him to consult at a healthcare center, from which he was referred to a reference center 12 hours after the incident with suspected severe arachnidism.
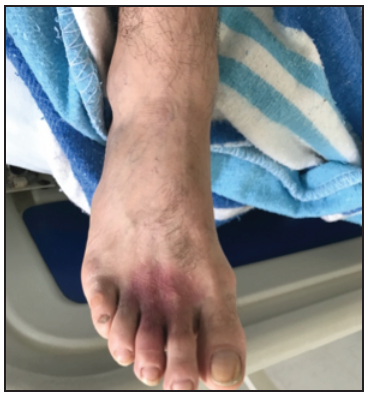
On admission, he was hemodynamically stable, with an approximately 5 cm erythematous macular rash on the dorsal skin at the base of the second and third toes (Figure 1), with pale borders and a violaceous center, scant blisters, desquamation and signs of local ischemia (delayed capillary refill). The initial management included intravenous fluids, analgesia, antihistamines and steroids. Antiarachnid serum was not administered due to the time elapsed since the inoculum and the fact that the admission paraclinical exams were normal (Table 1).
Table 1 Paraclinical tests on admission (+12 hours), at 48 hours, and on the fifth inpatient day.
| Exam | Admission | Day 2 | Day 5 |
|---|---|---|---|
| Hgb | 14.2 g/dL | 12.5 g/dL | 13.4 g/dL |
| Hct | 42.6% | 37.1% | 38.8% |
| WBC | 7,200/µL | 6,600/µL | 6,800/µL |
| Neutrophils | 88.5% | 62.1% | 57.2% |
| Platelets | 178,000/µL | 199,000/µL | 206,000/µL |
| BUN | 12.5 mg/dL | 11.6 mg/dL | 12.1 mg/dL |
| Creatinine | 0.75 mg/dL | 0.65 mg/dL | 0.81 mg/dL |
| Sodium | 138 mEq/L | 142 mEq/L | - |
| Potassium | 4.52 mEq/L | 3.65 mEq/L | - |
| PTT | 23.8 sec | 24.9 sec | 29.2 sec |
| PT | 10.7 sec | 10.8 sec | 12.1 sec |
| INR | 1.03 | 1.04 | 1.16 |
| Total bilirubin | 0.36 mg/dL | 0.24 mg/dL | - |
| Direct bilirubin | 0.18 mg/dL | 0.14 mg/dL | - |
| AST | 22.9 U/L | 17.4 U/L | 221.6 U/L |
| ALT | 25.3 U/L | 30.6 U/L | 410.0 U/L |
Hgb: hemoglobin; Hct: hematocrit; WBC: white blood cell count; BUN: blood urea nitrogen; PTT: partial thromboplastin time; PT: prothrombin time; INR: international normalized ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
Over the next 48 hours there was increased local pain and proximal progression of the edema. A Doppler ultrasound was performed finding a deep vein thrombosis in the proximal third of the posterior tibial vein and the distal third of the tibioperoneal trunk. Seventy-two hours later he developed liver dysfunction with transaminases increasing to hepatitis levels, no alterations on abdominal ultrasound or hepatic-portal-splenic Doppler, and negative hepatitis viruses. However, a de novo HIV diagnosis was made, confirmed by fourth-generation Elisa, with a viral load of 25,477 copies/ml, and a CD4 count of 517 cells, classified as a stage A1 HIV infection.
In the following weeks, antiretroviral and anticoagulant (rivaroxaban) therapy were started due to the thrombotic risk in HIV patients. The cutaneous lesion also resolved, and the hepatic enzymes normalized with no further interventions other than those already described.
Discussion
Spiders are cosmopolitan animals widely distributed in Latin America, with loxoscelism being relatively common in our setting 6.
Most (except Atrax robustus) avoid contact with man and have an inoculating apparatus which is poorly fitted for attacking large mammals. However, just 30 micrograms of protein in the venom can cause anywhere from mild local symptoms to death in susceptible patients (at the extremes of the life span or those with comorbidities) 7,8. Despite its clinical importance and relative frequency in the region, there is significant underreporting as it is not subject to mandatory reporting, which makes it difficult to obtain reliable epidemiological data 9.
More than 100 species of the Loxosceles genus have been described, which have the capacity to cause dermonecrosis through the action of sphingomyelinase D (SMaseD), an enzyme which induces endogenous metalloproteinases 10 whose cytotoxic effect causes hemolysis, kidney injury, coagulation disorders and hepatic damage. While SMaseD is the main component of the venom, other elements like histamine, hyaluronidase, alkaline phosphatase and metalloproteases have significant biological action as well 11, which has spurred a "venomous" focus in the search for specific treatments 12,13.
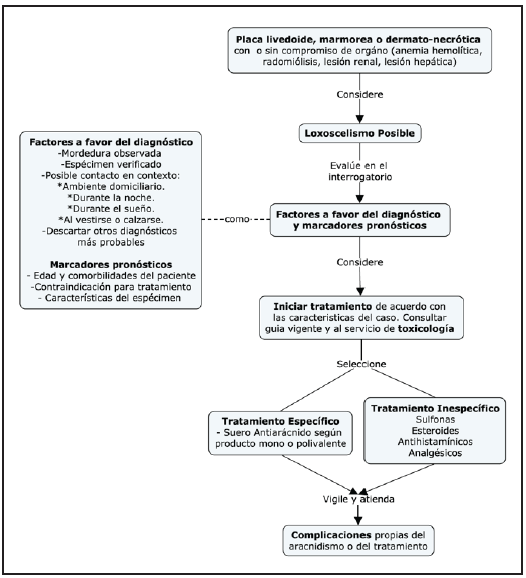
This entity is diagnosed clinically, based on the history reported by the patient. The English literature recommends capturing the arachnid to classify it morphologically, taking into account its small size (1-4.5 cm length), brown color in the shape of a violin, and three pairs of eyes arranged in three dyads 14. However, it is advisable to avoid re-exposure to poisonous animals to avoid new inoculations; therefore, a careful history is the basis for identifying presumptive cases and making an appropriate diagnosis (Figure 2). In loxoscelism, the bite tends to occur within the home, at night, while dressing or while sleeping, followed by progressively increasing lancinating pain 8,15,16. It should be noted that some cases do not have immediate local symptoms, and thus the bite may not be noticed, and the diagnosis is limited to identifying the cutaneous lesions and a compatible clinical course 17.
Loxoscelism is classified according to its manifestations as dermonecrotic (90%) when it is limited to the skin, producing pruritis, pain, local erythema, blisters and a "livedoid plaque" characterized by its ischemic violaceous center surrounded by a pale halo of vasoconstriction 15. On the other hand, the cutaneous-visceral manifestation includes rapid-onset (less than 24-hour) organ involvement; noting that 50% of the dermonecrotic manifestations are associated with fever, headache, vomiting, arthralgias and myalgias, without these implying cutaneous-visceral involvement 20. The latter requires paraclinical evidence of altered hemoglobin, lactate dehydrogenase and bilirubin levels secondary to intravascular hemolysis; coagulation disorders due to protein C dysfunction; or altered creatine kinase, blood urea nitrogen and transaminases due to rhabdomyolysis, kidney injury and hepatic damage, respectively 18,19.
This particular case had an atypical clinical presentation, given the late-onset cutaneous-visceral manifestation, probably related to the coexistence of HIV infection as a proinflammatory and procoagulant factor due to excessive production of cytokines like TNF-α, IL-1 and IL-6 which activate the coagulation system and decrease protein synthesis in the fibrinolytic system 25,26, which would favor (synergy) the hemostasis disorders attributable to loxoscelism 27,28. This evidences a possible differential response to arachnid venom in this particular population, a fact substantiated in previous studies on the contact between arthropods and patients infected with retroviruses 29.
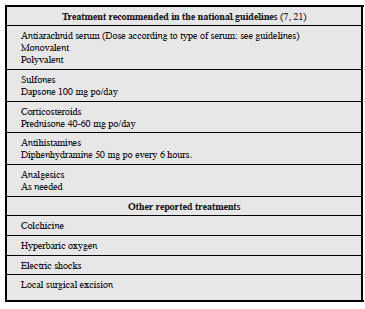
The treatment of arachnidism is currently controversial, due to the lack of clinical trials in the literature evaluating the proposed intervention (Table 2). At the moment, expert recommendations and interventions based on retrospective observational studies prevail. As a general measure, a minimum of 24 hours' observation is recommended in order to wash the site with sterile solution, immobilize in a functional position and provide tetanus prophylaxis. Analgesia and antihistamines should also be considered, as needed 7, along with prednisone (40-60 mg/day), dapsone (100 mg/day for anti-inflammatory purposes) and antiarachnid serum 21. It is interesting that the national guidelines for treating toxicological emergencies (2017) do not include a window period for the use of antiarachnid serum. In this regard, the literature has shown no benefit in administering it 12 hours after the inoculation, and further indicates that it may cause significant immediate and/or delayed immune reactions, which limit its use 22-24. Antiarachnid serum was not administered in our case as it was not available, and the consult occurred outside of the window period.
Arachnidism in Latin America continues to be an underestimated event today, even though its clinical importance and relative frequency have been described. Future research areas should include specific diagnostic tests which would detect the venom, to confirm presumptive cases, in addition to therapeutic alternatives to the current antiarachnid serum, seeking to decrease adverse responses and develop targeted therapies. We propose the production of monoclonal antibodies or the synthesis of target molecule inhibitors such as complement and adamalysine (a metalloproteinase activated by SMaseD). In addition, clinical trials must be carried out to evaluate the safety and effectiveness of the available therapeutic options. The use of chelating agents, like tetracyclines, heparin and ethylenediaminetetraacetic acid (EDTA), has even been proposed, which could be useful in preventing the spread of dermonecrosis by acting against the inoculated metalloproteinases. Until then, preventive measures for avoiding contact, and adherence to expert recommendations in the available guidelines, appear to be the wisest course.











 text in
text in