Introduction
Patients with end-stage renal disease (ESRD) may have a wide range of bone and joint disorders, including renal osteodystrophy, crystal-induced arthritis, avascular necrosis, soft-tissue calcification, amyloidosis, and bone infections, among others 1. Among these disorders, destructive spondyloarthropathy (DS) has been recognized as an entity found in patients with ESRD, which can lead to serious complications that put patients' functionality and life at risk 2. Below, we present the case of a patient on hemodialysis who consulted for thoracic pain and paraplegia, in whom we found thoracic vertebral body destruction secondary to destructive spondyloarthropathy associated with his chronic kidney disease.
Case presentation
A 70-year-old man with a six-year history of diabetic nephropathy and ESRD, on hemodialysis, consulted with a complaint of two weeks of intense thoracic back pain with lower limb paresthesias and inability to walk. The physical exam showed a patient with paraplegia and intense pain on palpation of the T5 and T6 vertebrae. Initially, infectious spondylodiscitis was suspected. The laboratory tests reported a creatinine level of 6.39 mg/dL, CRP 0.43 mg/dL, Hgb 10.8 gr/L, leukocytes 7,500, platelets 152,000, ESR 23 and negative blood cultures. The spinal x-ray showed a collapsed T5 vertebral body with anterior wedging and more than 50% loss of height, as well as loss of the T5-T6 intervertebral space (Figure 1). The computed tomography described T5-T6 as having marked irregularity and sclerosis of the intervertebral space, with end plate destruction. The resonance showed diffuse abnormalities in the intensity of the vertebral bodies, with hypointensity on STIR, loss of height and marked irregularity of the end plates and the T5-T6 disc, vertebral body edema, and retropulsion of the posterior wall towards the spinal canal which resulted in a narrowed, 3.5 mm canal with spinal cord compression (Figures 2 and 3). He underwent surgery in which a T5-T6 lesion was found, and paravertebral dissection to the laminae and transverse apophyses, along with T5 and T6 laminectomy, was performed. The spinal column was stabilized, and several samples were taken for culture and pathology. Antibiotics (piperacillin, tazobactam and vancomycin) were begun and then discontinued as all the surgical sample cultures (bone and disc) were negative. The histopathological study reported fragments of bone, cartilaginous and fibroconnective tissues with degenerative changes, chronic inflammation and reactive changes. Congo red and crystal violet staining was negative for amyloid. Infection or malignancy were ruled out and a metabolic bone disorder study was begun. Laboratory tests showed severe hyperparathyroidism (PTH 1,291 pg/mL), hyperphosphatemia (phosphorus 12 mg/dL), hypercalcemia (calcium 10.7 mg/dL), and elevated B2 microglobulin (18.3 mg/L), results which supported the diagnosis of DS secondary to hyperparathyroidism. Therefore, he underwent parathyroidectomy (3/4 of the parathyroid) with satisfactory results. The pathology report showed markedly hyperplastic parathyroid tissue. On his last assessment, his calcium-phosphorus profile had normalized, and he had paraplegia and chronic pain as sequelae, with no other complications.
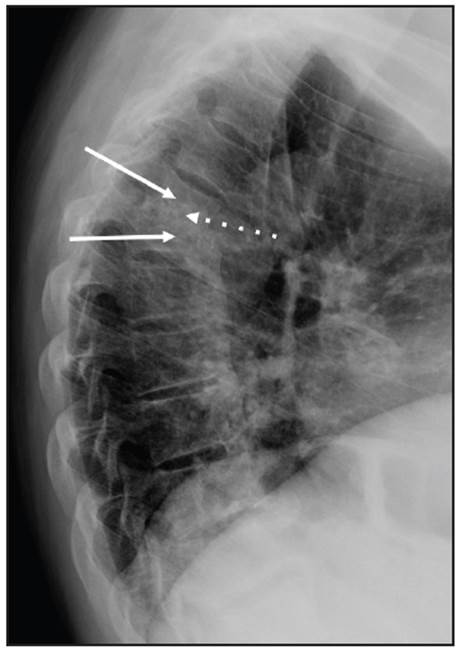
Figure 1 Lateral x-ray projection of the thoracic spine showing partial collapse of the T5 and T6 vertebral bodies (solid arrows), along with loss of the intervertebral space and sclerosis of the T5-T6 endplates (dotted arrow).
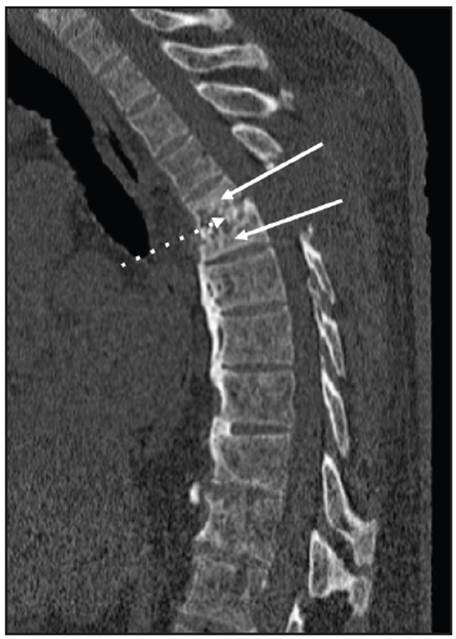
Figure 2 Sagittal reconstruction of the bone window thoracic spine tomography without contrast showing partial collapse of the T5-T6 vertebral bodies (solid arrows) and loss of the T5-T6 intervertebral space along with irregularity and sclerosis of the endplates (dotted arrow). These findings suggested spondylodiscitis.
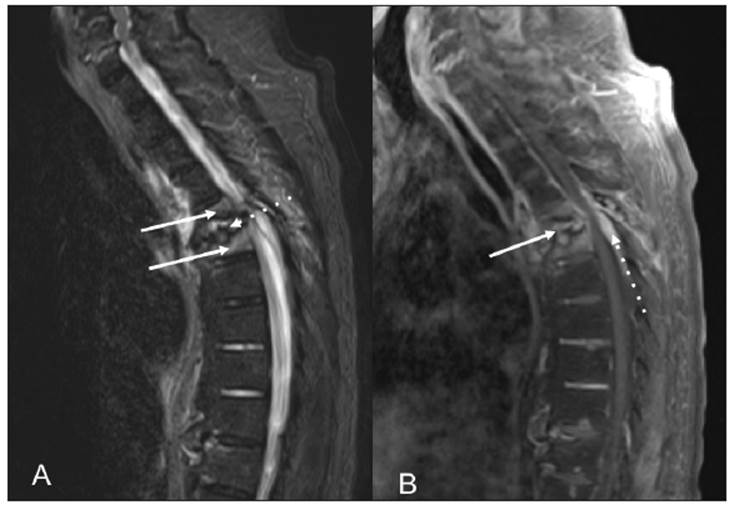
Figure 3 (A) Sagittal STIR sequence showing partial collapse and hyperintensity of the T5 and T6 vertebral bodies (solid arrows). Loss of height in the T5-T6 intervertebral space with endplate irregularity and intervertebral disc hyperintensity can also be seen (dotted arrow). (B) Sagittal Tl potentiated sequence with contrast and fat suppression showing enhancement of the intervertebral space and disc (solid arrow) and thickening and enhancement of the epidural space (dotted arrow). The findings were interpreted as spondylodiscitis with extension into the epidural space.
Discussion
We report the case of a patient with ESRD on hemodialysis who developed paraplegia due to spinal cord compression secondary to DS, a rare and possibly underdiagnosed complication in patients with ESRD. This disease occurs mainly in the lowest portion of the cervical spine, although it may also affect the craniocervical junction, as in the presented case 3-5, which may lead to death from brainstem compression 6-8.
The pathogenesis and pathophysiology of DS are not completely understood, and it has been related to the duration of kidney failure, duration of dialysis and bio-incompatibility of the dialysis membranes. Severe hyperparathyroidism leading to subchondral bone resorption has been suggested as a causal factor in vertebral abnormalities 9. There is also evidence indicating that β2-microglobulin amyloid deposits may contribute to destructive spinal changes in patients on chronic dialysis 10,11. In this report, we found severe hyperparthyroidism with a very high PTH, hyperphosphatemia and hypercalcemia as a factor associated with the bone destruction.
The prevalence of DS in patients with ESRD ranges from 5 to 25.3% 3,5,12,13. The average time from the onset of renal replacement therapy to DS is 9.6 years (14). However, some studies have reported this complication in the initial phases of kidney disease 15. In addition, cases of DS associated with chronic kidney failure without dialysis have been reported 16,17. The prevalence reported in various case series ranges from 12 to 34% in patients on hemodialysis and 10-39% in patients on peritoneal dialysis 15,18,19. However, it is difficult to compare its prevalence between the two dialysis modalities as it is uncommon for patients to remain on peritoneal dialysis for more than a decade.
Different imaging studies have been used to evaluate patients with suspected spondyloarthropathy. Due to their high spatial resolution and low cost, x-rays are considered to be the test of choice for the initial assessment of spinal abnormalities. The radiographic characteristics of DS consist of narrowing or obliteration of the intervertebral disc spaces, subchondral bone erosion or resorption in the superior and inferior endplates of the vertebral bodies, with or without cystic lesions, subchondral bone sclerosis and minimal osteophytosis 12,14,20,21. In addition, single or multiple spinal levels may be affected, and the lesions may involve adjacent or distant vertebral bodies 3,22. Early vertebral abnormalities may mimic those of early ankylosing spondylitis 14,22. With disease progression, the destructive lesions may mimic infection (spondylodiscitis) with disc space narrowing and irregularity and sclerosis of the subchondral bone of adjacent vertebral bodies. In advanced stages of the disease, the vertebral body fractures and collapses, and subluxation or spondylolisthesis may be found 2,12,20.
Computed tomography is the best way to detect small osteolytic areas in cortical bone, assess the distribution and extension of the destructive changes 21,23, and rule out abscesses. The tomographic findings which suggest infectious spondylitis include narrowing of the intervertebral disc space, irregularity of the adjacent vertebral end plates and a prevertebral soft tissue mass 24,25. Tomography may also be useful for showing vertebral subluxation and evaluating spinal compression 26.
Bone scans are highly sensitive for detecting acute bone infection, although they are nonspecific regarding the exact nature and extension of the infection 27. Technetium-99 bone scans have been used to visualize periarticular amyloid deposits, although they do not differentiate between amyloid-induced arthropathy and arthropathies from other sources 27. Iodine-131 or indium-111 scintigraphy radio-labeled with B2 microglobulin amyloid precursor protein may produce more reliable results 28,29. Magnetic resonance stands out for its high sensitivity in detecting the presence and extension of bone, joint and soft tissue infections 30,31.
The differential diagnosis of DS includes metastatic lesions, multiple myeloma, ossification of the posterior longitudinal ligament and cervical spondylosis 32. In metastatic disease, the lesions are more diffuse and less circumscribed. Multiple myeloma may be differentiated from spondyloarthropathy secondary to dialysis-related amyloidosis through serum and urine protein electrophoreses. A bone scan may be used to detect other myeloma lesions, for which magnetic resonance may also be useful. If spondylodiscitis is suspected, as in the reported case, the literature has also described the need for a biopsy of the lesions, which may be taken by needle aspiration or through surgery, with surgery providing the most consistent information. However, surgery has a higher morbidity than aspiration, which is known to be a safe method 13.
The treatment of DS is based on symptom control, orthopedic or surgical stabilization of the spine, and treatment of the underlying cause. In patients with spinal cord involvement, surgical decompression and spinal stabilization are paramount, always evaluating the risk/benefit, given the high surgical risk of patients with ESRD on hemodialysis 33. Surgical problems are not only related to surgical site complications such as susceptibility to bacterial infection or severe bone fragility, but also to general conditions like anemia, fluid and electrolyte disorders and blood pressure control. Multidisciplinary treatment is recommended for these patients to prevent intra and postoperative complications 34.
Given the pathophysiological correlation between DS and amyloid deposits, a change in high-flow membranes has been proposed as a strategy for decreasing the accumulation of beta-2-microglobulin, but this has not been validated. In patients with proven hyperparathyroidism, parathyroidectomy is indicated, which prevents complications and future lesions. This was the case of the reported patient who required this surgery due to severe hyperparathyroidism. Kidney transplantation should also be considered as an effective treatment strategy, as this resolves the problem of amyloid deposits and metabolic bone disorders which are part of this disease's pathophysiology 1).
In conclusion, DS is a complication of advanced chronic kidney disease with a variable clinical presentation, and its prevalence is 10-15% of the population on dialysis. The duration of dialysis and advanced age are the strongest predictors of its occurrence. It is diagnosed based on imaging findings of severe narrowing of the disc space and erosion of adjacent vertebrae without significant osteophytosis. However, when the diagnosis is not clear, a histopathological study is essential for ruling out infections and malignancies. The greatest clinical challenge is to differentiate it from an infectious process. The treatment goal is to improve the symptoms and prevent neurological deficits. As patients with end-stage renal disease live longer today, it is expected that this complication will be seen more frequently.











 text in
text in 


