Introduction
Olmesartan is an angiotensin II type 1 receptor blocker usually used to treat patients with hypertension. Recently, several cases of sprue-like enteropathy associated with the use of this medication have been reported after months or even years of treatment 1. The most common symptom is severe chronic diarrhea with weight loss, and there may also be nausea, vomiting and abdominal pain. Complications may include severe dehydration, acute kidney failure, hypoalbuminemia and electrolyte abnormalities, leading to hospital admission 2.
Clinical case
This was an 80-year-old male with a several-year history of essential hypertension and dyslipidemia. He was being followed by cardiology due to sclerotic hypertensive heart disease, severe aortic stenosis and moderate aortic regurgitation, and had been taking olmesartan medoxomil/ hydrochlorothiazide 40/25 daily for his hypertension, for more than four years.
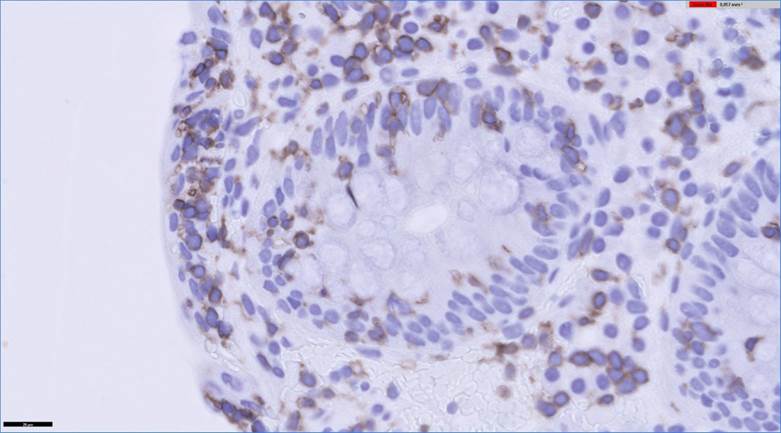
The patient complained of diarrhea for the last four months consisting of 10-15 liquid stools per day with no clear pathological products. The clinical picture was accompanied by a 6 kg weight loss over the last month and a half. Prior to consulting in the emergency room of our hospital, he had been seen several times by his primary care doctor, who eventually prescribed ciprofloxacin and loperamide, with no improvement. On his arrival in the emergency room, he had nonspecific dizziness and felt ill. Remarkable findings on physical exam included hypotension (60/40 mmHg), skin pallor, a positive skin pinch test, and oligoanuria, all of this in the context of hypovolemic shock secondary to gastrointestinal losses, requiring treatment under observation in the emergency room. His tests showed: urea 127 mg/dL, creatinine 10.91 mg/dL, sodium 144 mEq/L, potassium 2.90 mEq/L and C-reactive protein 33.7 mg/L. Venous gases were remarkable for a pH of 7.04 and bicarbonate of 13.9 mmol/L. An emergency abdominal ultrasound showed kidneys with a normal size, echostructure and parenchymal thickness, and ruled out urinary tract obstruction. Once his clinical situation was stabilized, an exhaustive study was carried out, with the following results: liver function, vitamin B12, folic acid, thyroid hormones, basal cortisol, total serum protein, stool cultures, stool studies for parasites, Clostridioides difficile toxin in the stool, blood cultures, antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA) and anti-transglutaminase IgA antibodies within normal limits. In light of these results, the case was consulted with the gastrointestinal (GI) service, with the decision made to carry out an upper GI endoscopy and ileocolonoscopy. The endoscopies were remarkable only for multiple sigma diverticula, with no other clear macroscopic lesions. The pathological anatomy studies of the biopsies from the second portion of the duodenum showed very unevenly distributed atrophy and shortening of the villi along with very irregular intraepithelial lymphocytosis (10 to 20 lymphocytes per 100 enterocytes evaluated by CD3) with almost no involvement of the villous apices, all probably related to chronic olmesartan use (Figure 1). In the colon, multiple biopsies led to the diagnosis of moderate lymphoplasmacytic pancolitis, suggestive of olmesartan-induced sprue-like enteropathy (Figure 2).
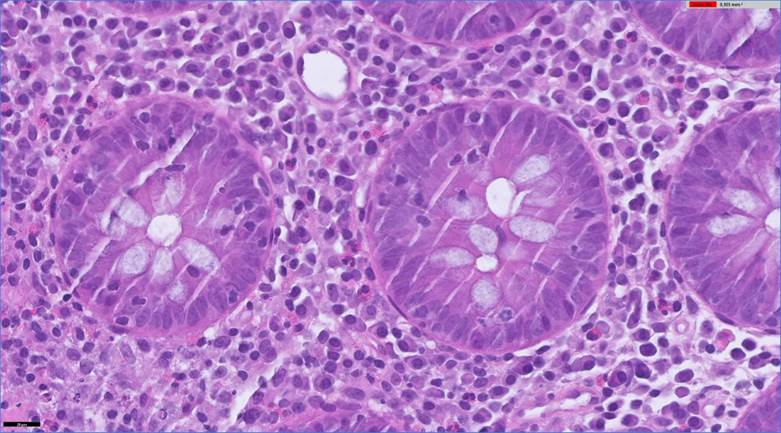
Figure 2 Colon biopsy. Hematoxylin-eosin 600x, intraepithelial lymphocytosis and inflammatory lymphoplasmacytic infiltrate.
In the absence of diagnostic criteria for celiac disease, with the biopsy results, and having ruled out other organic diseases as the cause of the patient's signs and symptoms, olmesartan use was determined to be the most probable cause of the enteropathy, and thus was discontinued. Once olmesartan was discontinued, the patient recovered from the diarrhea, regained weight, and remained asymptomatic on follow up.
Discussion
In 2012, Rubio-Tapia et al. 3 described the appearance of sprue-like enteropathies closely related to olmesartan use, which has since been reinforced by the United States Food and Drug Agency. Enteropathy associated with other sartans appears to be quite rare 4.
The causal mechanism of this disease is currently unknown 3. Due to its pathophysiology, the signs and symptoms do not appear immediately after introducing the medication; rather, the duodenal epithelium must be changed over a period of months or even years 1,5. The histological changes described in olmesartan-induced enteropathy may range from intraepithelial lymphocytosis and lymphocytic proliferation of the lamina propria to marked villous atrophy 4, which were found in the biopsies of the patient we are presenting. Growth factor-beta (an important factor in maintaining intestinal immune homeostasis) inhibition by angiotensin II receptor blockers 6 has been described in the literature. Whether this mechanism may be related to the duodenal villous atrophy found in these patients is unknown. Treatment for this disease includes discontinuing the medication and, in severe cases, oral or intravenous steroids have mitigated the symptoms 7. In the case presented, the medication was discontinued, and low-dose steroids were begun for seven days, with progressive improvement of the diarrhea.
Thus, we believe it is necessary, especially with the increased prevalence of this entity over the last few years, to include it in the differential diagnosis of chronic diarrheal syndromes of unclear etiology 8. Since the associated complications may potentially be serious, an exhaustive review of the patient's chronic pharmacological treatment must be included in the medical history.











 text in
text in