Introduction
The COVID-19 pandemic has caused high overall mortality; vaccination is the only effective strategy for decreasing morbidity and mortality. However, this vaccination is not risk-free 1-2.
COVID-19 vaccine-related immune thrombocytopenia is rare. There are few described reports and its diagnostic criteria have not been clearly validated. Therefore, we decided to compile this case to contribute to the recognition of this type of adverse event.
Case presentation
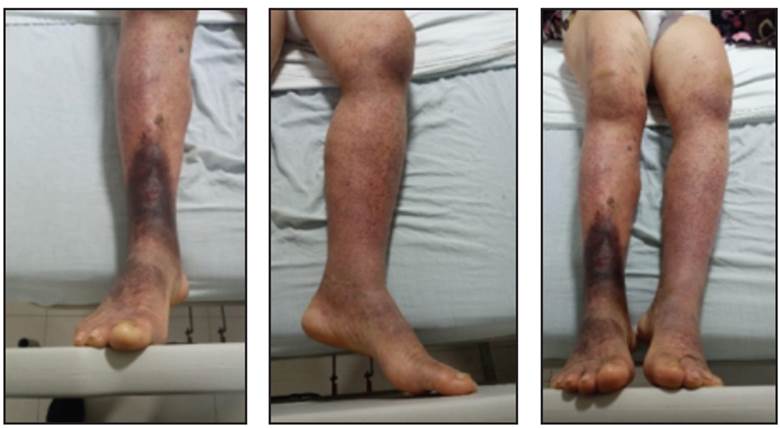
This was a female patient in her seventh decade of life who was admitted for purpuric lesions on her lower limbs which began on the fourth day after her second dose of the AstraZeneca COVID-19 vaccine and ascended progressively towards her trunk, associated with arthralgias, abdominal pain and nausea. She was admitted with a platelet count of 13,000. Her medical history was remarkable only for high blood pressure being treated with losartan 50 mg/twice daily. She denied taking antiplatelet or anticoagulant medications. On physical exam she was hemodynamically stable, her vital signs were within normal limits, she was afebrile, and she had no neurological deficits, no chest abnormalities and no organomegaly or abdominal abnormalities. Scattered purpuric lesions were found on the skin of the lower limbs, ascending to the trunk, which were less than 5 mm in diameter, and non-blanching (Figures 1-3).
COVID-19 swab tests were negative. During her hospitalization, antinuclear antibodies and anti-double-stranded DNA antibody levels were ordered, looking for collagen disease. She also had blood chemistry, kidney and liver function and electrolyte tests ordered during her follow up; D-dimer levels were greater than 10,000. At 48 hours, her platelets were at 8,000, and at 72 hours they fell to 5,000; she had no bleeding episodes during her stay.
Her immunoglobulin levels were within normal limits. Serology tests for hepatotropic, herpes, HIV and Epstein Barr viruses were negative. In light of the broad repertoire of tests ordered, all of which were negative, the trigger of the immune thrombocytopenia was determined to be the vaccination four days prior to the onset of the whole clinical picture. She required corticosteroids with no improvement, and therefore was begun on five days of immunoglobulin at I g/kg/day times three doses, with 27,000 platelets after 48 hours of treatment. After eight days of hospitalization, the subsequent platelet counts were above 50,000, and she was discharged after 13 days with 11,400 platelets for ongoing management by hematology.
Discussion
This case is anecdotal, given the rarity of COVID-19 vaccination-induced thrombocytopenia. The German Society of Thrombosis and Haemostasis discovered that out of the approximately 2.2 million doses of the AstraZeneca vaccine that had been administered at the time their article was published, a total of 31 cases of thrombosis had been reported. These occurred 4-16 days after vaccination, and thrombocytopenia was reported in 19 patients, with a fatal outcome in nine 3. In this type of events, a mechanism similar to that of heparin-induced thrombocytopenia has also been found in four patients in whom the antibodies caused platelet activation through the Fc receptor 3. The article by Schultz et al. 4 described five healthcare workers 32-54 years old who presented venous thromboembolism in unusual sites and thrombocytopenia, which occurred 7-10 days after vaccination. The patients had elevated D-dimer levels and high levels of IgG antibodies against platelet factor 4 (PF4) complexes, measured by ELISA. Treatment was begun with immunoglobulin and prednisolone, which increased the platelet count. In Greinacher et al.'s study 5, II patients from 22-49 years old had one or more thrombotic complications 5-16 days after the vaccination. All the patients had concurrent thrombocytopenia, with platelet counts ranging from 9,000 to 107,000. The serum test for all patients was positive for anti-platelet factor 4 antibodies.
The main hypothesis for the mechanism through which the vaccine triggers immune thrombocytopenia is related to the reaction between cationic platelet factor 4 and anionic free DNA. A 2013 study in a murine model showed that DNA and RNA form multimolecular complexes with PF4 and expose the epitope at which the anti-platelet factor 4 antibodies bind, causing an immune response similar to that of heparin-induced thrombocytopenia 6.
Various medical societies have published guidelines on how to deal with this adverse reaction 7. The following criteria are proposed for diagnosing thrombocytopenia following COVID-19 vaccination: the onset of symptoms within 4-28 days after vaccination, thrombocytopenia and elevated D-dimer. All patients with signs and symptoms suggesting thrombosis or thrombocytopenia 4-28 days after COVID-19 vaccination should have a complete blood count with coagulation tests; the levels of fibrinogen are reduced or even normal 8. The combination of thrombocytopenia and elevated D-dimer may be considered a sufficient criterion for immune thrombocytopenia following vaccination. The widely available platelet activation tests include platelet serotonin release, and the classic assay of heparin-induced platelet activation 9.
For anticoagulation, anticoagulants other than heparin should be used, including direct factor Xa inhibitors, direct thrombin inhibitors and fondaparinux. However, the effects of administering heparin to patients with this adverse event are still not clear, with some tests suggesting that heparin could inhibit antibody-mediated platelet activation specifically after COVID-19 vaccination. In patients who received intravenous immunoglobulin and prednisolone, the platelet count increased despite continuing heparin treatment 6. Similarly, serum samples from patients with thrombocytopenia after vaccination have been analyzed, finding that platelet activation was inhibited by the heparins.
A team at Birmingham University published the results of their study which looked at the effects of serum from patients with vaccination-induced thrombocytopenia on platelet aggregation and evaluated the usefulness of the available medications in preventing its activation. The authors found that low (0.1 and 0.3 U/mL) and high (100 U/mL) concentrations of heparin blocked in vitro platelet activation in serum samples obtained from patients with COVID-19 vaccination-induced thrombocytopenia (10). However, studies are needed to evaluate the role of heparin in vaccination-induced thrombocytopenia, as anticoagulants other than heparin have a greater risk of hemorrhage 11.
It is important to note that platelet transfusion is contraindicated unless the patient is to undergo an invasive procedure with a high risk of bleeding 12. Screening is generally done using heparin-induced thrombocytopenia ELISA, as the immunological mechanisms are very similar, and confirmation requires both ELISA and activation tests.
COVID-19 vaccination-induced thrombocytopenia is still possible if the ELISA test is positive but the platelet activation tests are negative.
A team at the University of Greifswald developed and evaluated the use of a rapid test for diagnosing COVID-19 vaccination-induced thrombocytopenia. The test, known as PF4-induced flow cytometry-based platelet activation (PIFPA), is a cytometric test based on an assay of washed platelets. The PF4-induced platelet activation test has a high specificity for antibodies related to the AstraZeneca vaccine, but for now is only found at specialized laboratories 13.
Following the administration of millions of doses of the various types of COVID-19 vaccines, after their emergency approval, the development of serious adverse events is closely monitored around the world. However, the recent appearance of a post-vaccination syndrome with similar characteristics to heparin-induced thrombocytopenia has raised some alarms, with several medical societies providing recommendations for the diagnosis and treatment of this syndrome, known as thrombocytopenia syndrome after COVID-19 vaccination.
Keeping in mind that the risk of this type of event continues to be extremely low, and the benefits of the vaccine exceed the risks, studies are needed to clarify the pathophysiology behind vaccine-induced thrombocytopenia, specifically for COVID-19, and create strategies to decrease the risk of adverse events.
Currently, the FP4-heparin ELISA assay continues to be the best tool for detection, while others have limited sensitivity and clinical usefulness.
Immune thrombocytopenia after COVID-19 vaccination is an area of constant study. For now, we must draw on the international societies' recommendations for its diagnosis and treatment. At the moment, there are limited available molecular tests in our setting; multidisciplinary participation is needed in this type of cases. This case is limited in not having been able to perform specific functional and molecular studies on this patient. The question which remains unanswered is which therapeutic strategies have the best results, as there are no studies with representative samples, and the available scientific evidence is of low quality.
Treatment with corticosteroids and immunoglobulin led to a recovery in the platelet count after three sessions of immunoglobulin. The ongoing treatment plan included prednisone adjusted to 1 mg per kg of body weight and follow up with hematology.











 text in
text in