Introduction
Spontaneous recovery from hemiplegia following a cerebrovascular accident (CVA) has an ontogenic pattern 1 that is accompanied by increased critical neurodevelopmental period proteins 1. Reopening of the critical periods has been proposed as a CVA treatment 2,3. It has been suggested that rTMS at 10 Hertz can reopen the critical periods in adult patients with amblyopia, 4. In patients with post-CVA hemiplegia, rTMS at 10 Hertz may reopen the critical periods and produce movement recovery with an ontogenic pattern.
Clinical case
This was a 38-year-old patient with a severe neurological deficit 17 months after experiencing a brainstem hemorrhage secondary to the rupture of an arteriovenous malformation, which did not require surgical treatment (Figure 1). The neurological exam showed an alert patient with normal language comprehension, severe dysarthria, minimal move ment of the uvula and soft palate, a very reduced gag reflex, right hemiplegia, left hemiparesis, a permanently flexed head, severe trunk instability and inability to stand. The patient communicated through an alphabet board used by her caregiver in response to blinking. A neurorehabilitation expert recommended the use of noninvasive brain stimula tion as an adjuvant to rehabilitation. She underwent rTMS for her CVA in an off-label treatment modality 5-7. This manuscript was approved by the ethics committees of Fundación Universitaria Sanitas (CEIFUS 1382-20), and Hospital Infantil de San José (113/2020).
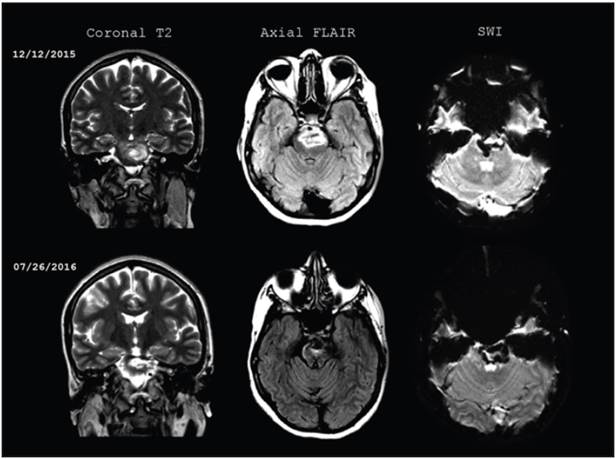
Figure 1 Initial brain magnetic resonance and follow up seven months after the CVA. Brainstem hemorrhage extending form the mesencephalon to the protuberance, with a heterogenous signal on T2, and perilesional edema on FLAIR. On SWI, the blood remains in different stages of progression, with no changes in size compared to the initial images.
First cycle
We adapted a bilateral 10 Hertz rTMS protocol for dysphagia 10, as the patient's severe motor deficit contraindicated the use of inhibitory stimulation (8, 9) and the patient could attend a maximum of three sessions per week. We administered 20 rTMS sessions using a MagPro R30 machine, a figure-eight coil (MCF-B65), and a frequency of two or three sessions per week. Each session began by locating the "hot spot" and determining the resting motor threshold (rMT). The rMT was based on observing a minimal muscle contraction. Due to the severe oropharyngeal motor deficit and constant head flexion, we used the upper extrem ity as the "hot spot." The "hot spot" was able to be located over the right primary motor cortex (M1) and the rMT was obtained from the left abductor pollicis brevis muscle. The magnetic pulse over the left hemisphere did not cause muscle contraction, and therefore we located the left "hot spot" in a homotopic M1 site and used the right rMT. The rTMS was applied over the right Ml in five-second trains at 10 Hertz frequency and an intensity of 90% rMT, with a 55-second interval between trains, for 15 minutes and a total of 750 pulses. This was followed by the same protocol on the left M1. After this cycle, the patient had improved swallowing and, unexpectedly, voluntary contraction of the right rhomboid muscles.
Second cycle
No further improvement was seen after two months of re habilitation, and a second cycle was applied. A cone-shaped coil (D-B80) was added to stimulate the motor cortex of the lower limbs. This coil was placed on the medial sagittal plane, wherever the greatest contraction of the left anterior tibialis muscle was seen. Ten-second rTMS trains were applied at 10 Hertz and 90% rMT, with a 50-second interval between trains, for 20 minutes and a total of 2,000 pulses 11. The frequency of the sessions was the same as in the first cycle, and the sessions alternated between the first cycle protocol and the lower limb protocol. At the beginning of this cycle, the routine search for the right hemisphere "hot spot" caused muscle contraction of the right paralyzed arm; that is, the ipsilateral motor evoked potential (iMEP) was seen. A few days later the patient began to have voluntary proximal movement of the right upper and lower limbs. After 14 sessions, proximal movement recovery was significant and magnetic stimulation was discontinued. One month later, the patient was able to hold up her head, maintain a sitting position and stand on her own (Figure 2).
Third cycle
Four months after the second cycle, the patient continued to have a paralyzed right hand, did not have dynamic equi librium while standing, and a third cycle of 20 sessions was conducted using the same parameters as the second cycle. In this cycle, the routine search for the left M1 "hot spot" caused right hand contraction; that is, the motor evoked potential (MEP) appeared. At the end of this cycle, the pa tient had slight voluntary movement of the right abductor pollicis brevis muscle.
Discussion
An adult patient with a chronic brainstem CVA improved her hemiplegia after receiving three rTMS cycles, which were initially aimed at treating dysphagia.
At the end of the first cycle, swallowing improved and voluntary movement unexpectedly appeared in the right rhomboid muscles. Swallowing is the model for studying the reorganization of axial muscle motor control following a CVA 12. Axial muscles are innervated by the corticore-ticulospinal tract (CRST) 13. We applied bilateral rTMS over M1, the site from which part of the CRST originates 13. Thus, we suspect that the appearance of voluntary axial movement was caused by CRST activation 14.
With the second cycle, the iMEP was visualized in the paralyzed arm, followed by recovery of proximal move ment and postural control. This confirmed our suspicion of CRST activation 14, since this tract is responsible for iMEP 15,16 and innervates the axial-proximal muscles responsible for postural control 13. In adults, iMEP is subject to cortical inhibition 16. Cortical disinhibition al lows latent pathways to be unmasked 17. We speculated that cortical disinhibition produced by rTMS at 10 Hertz 18 was responsible for unmasking the CRST ipsilateral to the hemiplegia. This supports the proposed therapeutic effect of the ipsilateral CRST for proximal movement rehabilita tion 19 and the contraindication for applying inhibitory ipsilateral stimulation in patients with severe CVA 8,9, as it inhibits the CRST 9.
Ontogenically, the iMEP disappears at age 10 due to inhibitory processes 20. In adult patients with severe mo tor deficits following a CVA, CRST disinhibition causes the iMEP to reappear 21, but this is associated with a poor prognosis 21. The opposite occurs in hemiplegic adolescents, in whom the appearance of the iMEP marks the beginning of recovery 22. Children recover better than adults after a CVA, due in part to brain plasticity 23. Young brains have greater plasticity because they have less cortical inhibition than adults 24. Cortical disinhibition increases and rejuvenates brain plasticity by reopening the critical periods 25. The rTMS at 10 Hertz causes cortical disin hibition 18. It has been suggested that rTMS at 10 Hertz causes a reopening of the critical periods 3,4, as it improves adult patients with amblyopia 4. In addition, rTMS at 10 Hertz over M1 increases the expression of c-fos and zif268 proteins 26, whose genes participate in the critical periods 27. We speculate that, in our patient, the rTMS at 10 Hertz over M1 reopened the critical periods and increased brain plasticity at a level which allowed the iMEP to appear and recovery to commence, as occurs in adolescents 22. This would support critical period reopening as CVA treatment 2,3, and leads us to suggest that the poor prognosis of iMEP in adult patients with severe CVA 21 could be related to insufficient brain plasticity, and that cortical disinhibition with rTMS at 10 Herz could help resolve it.
The MEP of the paralyzed hand appeared during the third cycle, followed by the onset of minimal distal voluntary movement, events related to the corticospinal tract (CST) 15. Corticospinal tract activation using high-frequency rTMS has a PLP-like effect 5-7. We have speculated that our patient's recovery began with an axial-proximal movement due to ipsilateral CRST unmask ing and continued with distal movement due to CST PLP. Unmasking and PLP are two brain plasticity mechanisms which require cortical disinhibition 17,28. Thus, we support the idea that cortical disinhibition maximizes the efficiency of the corticospinal tracts of patients with CVAs 5,29, allowing effective cerebral reorganization 5,29. We also support the idea that cortical disinhibition is a novel treatment strategy for CVAs 30, and suggest that it can be achieved by applying rTMS at 10 Hz.
Ontogenically, distal movement is produced only after there is an adequate proximal movement 31. This ontogenic pattern also occurs during spontaneous recovery from post-CVA hemiplegia 1 and was the pattern we obtained in our patient. In 2008, the London group proposed that recovery from a major CVA requires phased recruitment of the ipsilesional M1 and the contralesional premotor area 29, and that the latter is not required for recovery from a minor CVA 29. Our patient had a severe axial-proximal motor deficit and therefore required CRST recruitment. Most of the CRST fibers originate from the premotor area 13. We speculate that recruitment of the premotor area depends on whether there is adequate axial-proximal move ment, and thus the London group's proposal would be the neurophysiological correlate of the ontogenic post-CVA recovery model 29.
This study has several limitations. First, since only one case is reported, spontaneous recovery cannot be ruled out, nor can the placebo effect be ruled out in the absence of blinding. In addition, we did not conduct neurophysiological or tractography studies, nor did we systematically obtain the iMEP. Future studies are needed with a detailed evaluation in a larger sample size to confirm these findings.











 text in
text in 



