Introduction
Heart failure (HF) is defined as a clinical syndrome characterized by symptoms like dyspnea, edema and fatigue, and physical exam findings like peripheral edema, elevated jugular venous pressure and pulmonary rales. It occurs due to structural or functional abnormalities leading to decreased cardiac output and elevated intracardiac pressures at rest or during exercise, and should be treated according to its etiology 1.
Heart failure is classified according to its ejection fraction, as follows: normal left ventricular ejection fraction (LVEF) or preserved ejection fraction (pEF) is considered to be >50%, and reduced LVEF (rEF) <40%.
Patients with an LVEF between 40 and 49% are said to have mid-range ejection fraction (mrEF); however, in this paper we will review studies with an LVEF >40% 1.
Approximately 20 million people are thought to have pEF heart failure, with a 2% prevalence in industrialized countries 2. There is a higher incidence in males; however, prevalence is greater in women. In addition, one out of six patients over the age of 65 admitted to the emergency room have pEF heart failure 3.
The population of patients with pEF has a greater prevalence of prior conditions like hypertension, atrial fibrillation and, less frequently, ischemic heart disease 4.
Heart failure with pEF may be primary, as occurs with hypertrophic cardiomyopathy, or secondary to various etiologies such as hypertension or restrictive cardiomyopathy related to infiltrative disorders, amyloidosis and sarcoidosis 5.
The diagnosis of heart failure with pEF continues to be a challenge, requiring the following conditions: signs or symptoms of heart failure; preserved ejection fraction, defined as an LVEF > 40%; elevated concentrations of natriuretic peptides (BNP > 35 pg/mL or NT-proBNP > 125 pg/mL) and objective data of other underlying functional or structural cardiac abnormalities.
Structural abnormalities include a left atrial volume index >34 mL/m2 or a left ventricular mass index = 115 g/m2 (men) or 95 g/m2 (women) 6.
The goal of treatment is to decrease mortality and hospitalizations, and improve the quality of life and functional capacity. The treatment of patients with pEF is based on diuretics, beta blockers, mineralocorticoid receptor antagonists, angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) 7.
To date, these treatments have not been proven to reduce morbidity and mortality in patients with pEF. This could be related to the study population, consisting mostly of older adults with concomitant illnesses and comorbidities which contribute to the development of this condition and increase the risk of complications 8.
From this perspective, the objective of this review is to evaluate the effectiveness and safety of these medications in the different studies of heart failure with pEF in terms of mortality, hospitalization and improved quality of life, to contribute new evidence-based scientific knowledge.
Data collection
Method: a systematic review of the literature.
Criteria for including studies in the review
Types of studies
Included studies: randomized clinical trials. Excluded studies: cluster clinical trials or before-and-after, quasi-experimental or crossover studies.
Types of participants
Studies of patients over 18 years of age diagnosed with heart failure with an ejection fraction between 40 and 50% and >50%, with NYHA functional class II, III, or IV were included.
Studies of the following populations were excluded: pregnant patients, patients with terminal cancer, patients with chronic kidney disease with a GFR <30 mL/min, patients with acute heart failure and patients with acute myocardial infarction.
Types of interventions
Studies with any type of treatment for heart failure with pEF were included; however, the intention was to include the following types of medications:
Beta blockers: nebivolol, metoprolol, bisoprolol, carvedilol.
Angiotensin converting enzyme inhibitors: captopril, perindopril, quinapril, ramipril, enalapril, lisinopril.
Angiotensin II receptor blockers: candesartan, irbesartan, losartan, valsartan.
Loop diuretics: furosemide.
Mineralocorticoid receptor antagonists: spironolactone, eplerenone.
Neprilysin inhibitors/angiotensin antagonists: sacubitril/valsartan.
If current inhibitors: ivabradine.
SGLT2 inhibitors: dapagliflozin, empagliflozin.
Types of outcomes
Mortality: defined as the death rate in a population over a period of time from a specific cause, during the first 30 days, 60 days, 12 months and two years (from all causes-cardiovascular causes).
Quality of life: defined as the person's perception of his/her life situation, using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) or any other questionnaire reported by the included article.
Hospitalization: defined as the admission of a sick person to the hospital for decompensated heart failure, less than twice a year.
Adverse events: any adverse event during treatment such as heartburn, allergies, cough, atrioventricular block, electrolyte disorders or acute kidney injury.
Search strategy
To answer the research question, a combination of controlled (MeSH, Emtree, DeCS) and free terms (to consider spelling variants, synonyms, acronyms and truncations) were used, with field labels (title and abstract), proximity operators (ADJ) and Boolean operators (OR, AND).
The search was performed from June 10, 2020, through January 18, 2022, including articles from 1998 to 2022.
We specifically searched the following databases: The Cochrane Central Register of Controlled Trials, Ovid platform MEDLINE, Ovid platform: inception to June 2018 Ovid platform; MEDLINE Daily Update, Ovid platform; Embase,embase.complatform; and LILACS, IAHx interface.
Study selection
The authors independently selected the studies by title and abstract and then full text. If there was disagreement, a third author made the decision to include or exclude the studies.
Data extraction and critical appraisal were performed independently by three authors and then consolidated to write the article.
Results
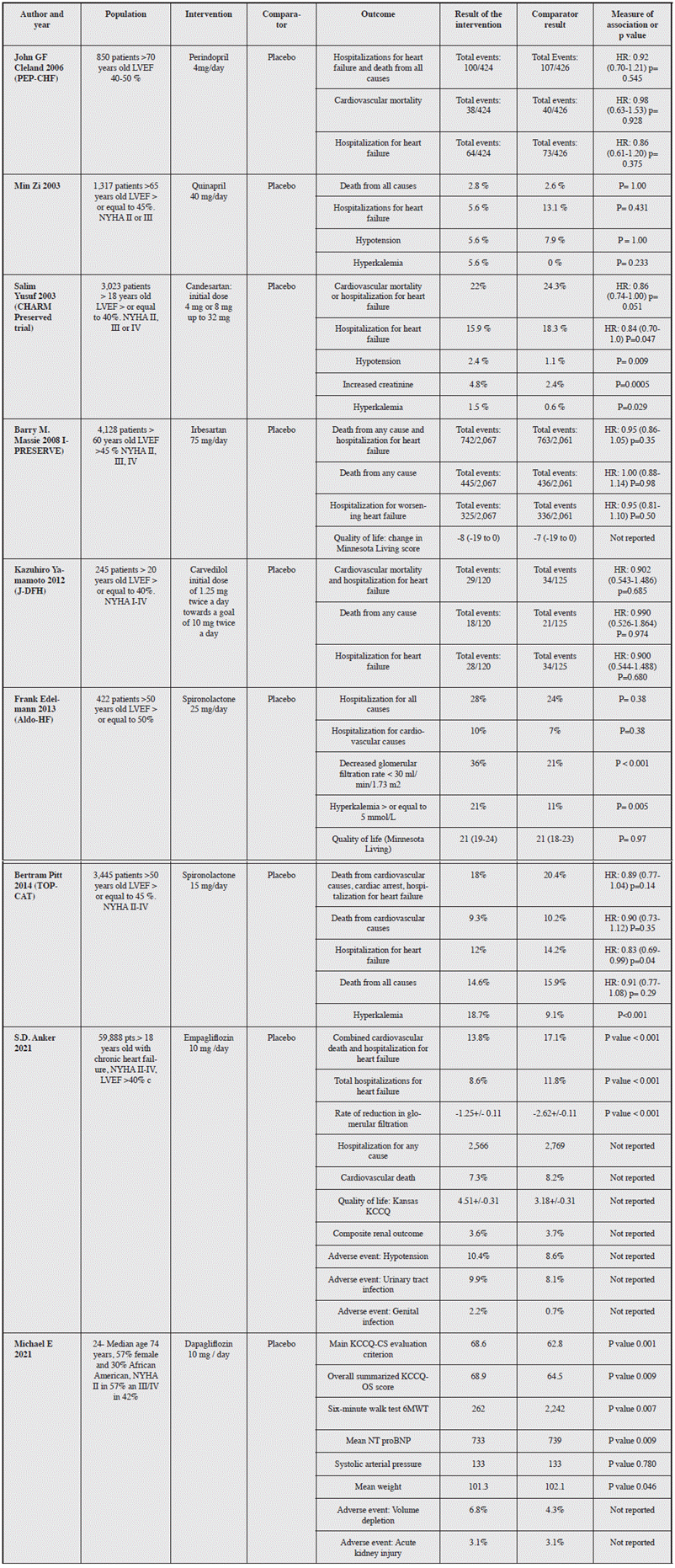
This systematic review was able to find 2,732 articles which were analyzed to determine their eligibility based on their title and abstract. After eliminating duplicates (111) and those excluded based on the criteria (2,572), 53 full-text articles were evaluated. Of these, 35 articles were excluded and 18 articles were ultimately included for qualitative synthesis.
These results will be discussed by drug group below (Figure 1).
Figure 1 PRISMA flowchart of the identification, eligibility and inclusion of articles for the systematic review of treatment for heart failure with preserved ejection fraction.
ACE inhibitors and ARBs
John G.F. Cleland's 2006 study (PEP-CHF) is a clinical trial which included 850 patients with heart failure and an LVEF between 40 and 50%, comparing the use of perindopril 4 mg/day vs. placebo and evaluating a composite primary outcome: hospitalizations for heart failure and death from all causes, finding no significant differences between the two groups (HR 0.92; 95% CI (0.70-1.21) p= 0.545) 9.
Min Zi's 2003 s tudy is a double-blind clinical trial which included 74 patients over the age of 65 with heart failure with an LVEF greater than or equal to 45%, NYHA II or III. It compared quinapril 40 mg/day with a placebo. After six months' follow-up, the following results were found:
Mortality from all causes was 2.8% for the quinapril group and 2.6% for the placebo group, p= 1.00. For quality of life, there were no statistically significant differences between the groups (p=0.804).
Regarding adverse events, there were no significant differences in electrolyte disturbances. One patient in the placebo group and six patients in the quinapril group developed a cough, which was not significant (p =0.053) 10.
Salim Yusuf's 2003 study (CHARM-Preserved Trial) is a randomized clinical trial which included patients with heart failure with LVEF greater than or equal to 40%, NYHA II, III or IV, comparing candesartan at an initial dose of 4 mg and 8 mg/day against placebo. After three years' follow-up, the study found that:
There was no significant difference in the composite outcome of cardiovascular mortality and hospitalization for heart failure, with an HR of 0.86 (0.74-100) p= 0.051.
For adverse events, creatinine was elevated in 4.8% of the candesartan group vs. 2.4% of the placebo group, p= 0.0005, and hyperkalemia occurred in 1.5% of the candesartan group versus 0.6% of the placebo group, p =0.029. 11
Barry M. Massie's 2008 study (I-PRESERVE) is a randomized clinical trial which included 4,128 patients with heart failure and an LVEF greater than or equal to 45%, NYHA II-IV, comparing irbesartan 75 mg once a day with progressive increments up to a maximum dose of 300 mg/ day against placebo. Four years' follow up was conducted.
The composite outcome of mortality from any cause and hospitalization for cardiovascular reasons showed no statistically significant difference (HR 0.95; 95% CI, 0.86-1.05; p = 0.35).
Regarding quality of life, after six months, the Minnesota Living with Heart Failure scale scores improved for both groups, but the difference in the degree of change between the two groups was not significant.
The difference between the two groups in serious adverse events due to hypotension, kidney dysfunction and hyperkalemia was not significant 12.
ACE inhibitors/ARBs plus diuretic
G W K Yip's 2008 study is a randomized clinical trial which included 150 patients with heart failure and an LVEF greater than or equal to 45%, NYHA II, II or IV, who were randomly assigned to diuretics alone, diuretics plus irbesartan or diuretics plus ramipril. The study found that the quality-of-life score according to the MLHFQ improved similarly in the three groups over 52 weeks (46, 51 and 50%, respectively, p= 0.01); however, when the groups were compared at 52 weeks, there were no significant differences (p=0.7) 13.
ARBs + neprilysin inhibitor
S.D. Solomon's 2019 study (PARAGON-HF) evaluated the use of sacubitril/valsartan in a population over the age of 50 with heart failure with an LVEF ≥ 45%, comparing twice daily drug administration (sacubitril 97 mg/ valsartan 103 mg) vs. valsartan (160 mg twice a day), finding that:
There was no difference in the compound outcome of mortality from all cardiovascular causes plus hospitalization for heart failure (RR 0.87; 95% CI: 0.75-1.01; p = 0.06). However, out of the 12 subgroups analyzed, four showed heterogeneity in the intervention, indicating a benefit in patients with an ejection fraction between 45-57% (RR 0.78; 95% CI: 0.64-0.95), females (RR 0.73; 95% CI: 0.59-0.90), those over the age of 65 (RR:0.85; 95% CI: 0.73-0.99), and in patients who were also receiving mineralocorticoid receptor antagonists (RR: 0.73; 95% CI: 0.56-0.95).
No differences were found in the incidence of death from all cardiovascular causes (HR: 0.95; 95% CI: 0.79-1.16).
No differences were found between the groups in total hospitalizations for heart failure (HR: 0.85; 95% CI: 0.72-1.00).
Adverse events: the compound renal outcome defined as death from kidney failure and decreased kidney function was lower in the sacubitril/valsartan group (1.4%) compared with valsartan alone (2.7%) (HR 0.50; 95% CI: 0.33-0.77).
The quality of life outcome showed no differences between the groups using the change in clinical status score of the Kansas City Cardiac Questionnaire (KCCQ) (HR 1.0; 95% CI, 0.0-2.1 14.
Beta blockers
Dirk J. van Veldhuisen's 2009 study (SENIORS) is a clinical trial which included 2,111 men and women ≥ 70 years old with LVEF ≤ 35% and with LVEF ≥ 35%, comparing nebivolol at an initial dose of 1.25 mg/day with a goal of 10 mg/day against placebo, with 21 months of follow up. The study evaluated mortality from all causes and hospitalization for cardiovascular reasons, finding no differences in the primary outcomes between the two groups (p = 0.720) 15. Kazuhiro Yamamoto's 2012 study (J-DHF) is a clinical trial which included 245 patients ≥20 years old with heart failure and LVEF ≥40%, with NYHA functional class I-IV, comparing carvedilol at an initial dose of 1.25 mg twice a day toward a goal of 10 mg twice a day, with a mean dose of 7.5 mg/day, against placebo, with a median follow up of 3.2 years. The study evaluated mortality from cardiovascular causes and hospitalization for heart failure, finding no significant differences between the two groups (p = 0.6854) 16.
Diuretics-aldosterone inhibitor
In Frank Edelmann's 2013 study (Aldo-HF), 422 patients ≥ 50 years old with heart failure with an LVEF ≥ 50% were randomized to receive 25 mg of spironolactone vs. placebo, evaluating the following outcomes:
No differences were found between the groups in hospitalizations for all causes (p= 0.38), nor in hospitalizations for cardiovascular reasons (p= 0.38).
Adverse events: worsening GFR was evaluated, finding 36% of the cases in the spironolactone group vs. 21% of the cases in the placebo group (p= <0.001), along with elevated serum potassium increasing ≥ 5 mmol/L at any time (p=0.005), as well as relevant hyperkalemia (>5.5 mmol/L), finding no differences between the groups (P= >0.99).
Quality of life: evaluated using the MLHFQ found no differences between the groups (p= 0.97) 17.
Bertram Pitt's 2014 study (TOPCAT) evaluated a population of 3,445 patients over the age of 50 with heart failure with an LVEF ≥45%, comparing spironolactone once a day vs. placebo and evaluating the following outcomes:
No significant differences were found in the primary composite of death from all cardiovascular causes, cardiac arrest and hospitalization for heart failure (RR 0.89; 95% CI: 0.77-1.04 p=0.14), nor in mortality from cardiovascular causes (HR:0.9; 95% CI: 0.73-1.12).
The spironolactone group had fewer hospitalizations (HR: 0.83; 95% CI: 0.69-0.99 p=0.004).
There was 18.7% hyperkalemia in the spironolactone group compared with 9.1% in the placebo group (p=<0.01) (18).
Miranda Merrill's 2019 study, a sub-analysis of the TOPCAT study describing gender-based (male/female) differences, with women making up 882 of the 1,767 patients (49.9%), found that:
The composite primary outcome of cardiovascular death, cardiac arrest and hospitalization for heart failure occurred in 30% of the women in the spironolactone group and 34% of the men, with no significant differences found (p=0.15).
Cardiovascular deaths plus hospitalization for heart failure in men and women occurred in 25.1% of the spironolactone group and 29.5% of the placebo group, with no differences found (p= 0.84).
Cardiovascular deaths plus hospitalization for heart failure in men occurred in 34% of the spironolactone group and 29.5% of the placebo group, with no significant differences found (p=0.84).
Death from all causes in spironolactone treatment was 15.8% for women and 25.2% for men (p=0.02), with a reduction in the women's group (HR: 0.66; 95% CI: 0.480.90; p=0.01) compared to the men's group (p=0.68) 19.
Selective If current inhibitor
Michel Komajda's 2017 study is a randomized, double-blind clinical trial which included 179 patients with heart failure and an LVEF greater than or equal to 45%, NYHA III-IV, comparing ivabradine at an initial dose of 5 mg/ po/q 12 hrs. vs. placebo. The study evaluated three final co-primary points: echo-Doppler E/e' ratio, distance covered in the six-minute walking test (6MWT) and plasma concentration of NT-proBNP, which are not objectives treated in this review.
There were no statistically significant differences between the groups in the occurrence of adverse events such as arrhythmias, angina (p = 0.633) or treatment interruption due to the occurrence of adverse events (P= 0.261) 20.
SGLT2
S.D. Anker's 2021 study, EMPEROR-Preserved, is a multicenter, randomized, double-blind clinical trial which included 5,988 patients with heart failure with an LVEF >40% and a II-IV functional class, comparing empagliflozin at a dose of 10 mg/day vs. placebo, evaluating as its primary objective the composite of cardiovascular death and hospitalization for heart failure. The primary outcome was found in 415 patients (13.8%) in the empagliflozin group and 511 (17.1%) in the placebo group, with statistically significant differences at a 95% CI (0.69-0.90) (p< 0.001), with a 21% reduction in relative risk, which was shown in a lower risk of hospitalization for heart failure.
The total number of hospitalizations for heart failure was lower in the empagliflozin group than in the placebo group (407 vs. 541), which was statistically significant (95% CI, 0.61 to 0.88; P<0.001).
In the kidney component, the slope of the reduction in glomerular filtration rate over time was favored by empagliflozin, with a difference of 1.36 nfl/min/1.73 m2 between the two groups (95% CI 1.06-1.66; P <0.001).
Adverse events occurred in 19.1% of the empagliflozin group vs. 18.4% in the placebo group, with hypotension, urinary tract infections and genital infections being more frequent in the empagliflozin group 21.
In Milton Parker et al.'s 2021 study, the following results were found:
There was no statistically significant difference in hospitalizations for any reason (2,566 vs. 2,769; empagliflozin vs. placebo, respectively (CRI 0.93 [95% CI 0.85-1.01]; p=0.10); however, the empagliflozin group required 33% fewer intravenous diuretics (CRI 0.67 [95% CI 0.57- 0.79]; p <0.0001) 22.
There was a reduction in the total visits reporting an outpatient increase in diuretics (CRI 0.73 [95% CI 0.650.82]; P < 0.0001) 22.
The quality of life outcome using the KCCQ change in clinical status score in the empagliflozin study Health Status and Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction found an improved quality of life and health status score with a KCCQ score >5 23.
Michael E. Nassif et al.'s study was a multicenter randomized, double-blind, placebo-controlled trial in patients with heart failure with a preserved ejection fraction, in which 324 individuals participated (162 patients with dapagliflozin and 162 patients in the placebo group). The participants were evaluated using KCCQ-CS, with a 5.8 point improvement found in the health status and symptoms of patients with heart failure after 12 weeks of treatment, (49.4 vs. 38.2%; adjusted OR = 1.64 (95% CI: 0.98-2.75), p=0.06.
A 20.1 meter improvement in the six-minute walking test (95% CI 5.6-34.7), p=0.007; and weight loss (effect size, 0.72 kg [95% CI: 0.01-1.42]), p= 0.046.
Adverse events: 44 (27.2%) and 38 (23.5%) in dapagliflozin vs. placebo, respectively, including acute kidney injury and volume depletion 24.
Scott D Solomon's 2021 study (DELIVER) is an international, multicenter, randomized double-blind trial comparing the effect of dapagliflozin 10 mg/day vs. placebo. The primary objective was to evaluate the time elapsed until the first cardiovascular death or worsening heart failure, and the secondary objective was to evaluate all of these events, with dual analysis (that is, of the total population and of patients with LVEF <60%). This study will complement the DAPA-HF study in which the results of both studies were combined to evaluate the effect of dapagliflozin on the complete LVEF spectrum 25.
Kentaro Ejiri's 2019 study (MUSCAT-HF) is a clinical trial randomizing 190 patients to receive luseogliflozin 2.5 mg/day vs. voglibose 0.2 mg/three times a day. It showed that the degree of reduction in the concentrations of BNP after 12 weeks is not significant (percentage change -9.0% vs. -1.9%; proportion of change with luseoglifozin vs. voglibose, 0.93; 95% CI, 0.78-1.10; p= 0.26) 26.
In the evaluation of biases, the studies had a low risk of random sequence generation bias, except for Veldhuisen's 2009 study and Zi's 2003 study, which had an unclear risk. Regarding concealment, the studies generally had an unclear risk of bias because they mentioned the method but were not explicit. For participant and staff blinding, most had a low risk of bias, except for the Yamamoto 2012, Yip 2008, and Scott D. 2021 studies, which had a high bias due to being open-blind studies. Most studies had a low risk of an incomplete data outcome since they did not lose patients. Most of the studies had a low risk of reporting bias
Discussion
The results of this systematic review have been classified according to each pharmacological group used in the various controlled clinical trials in patients with heart failure with pEF. It is relevant to consider the heterogeneity of these studies, which is why a meta-analysis was not considered to be a viable option. In general, it can be concluded that the studies included in this systematic review are of moderate quality, due to a high risk of bias in the Yip and Yamamoto studies included in the ACE inhibitor/ARB plus diuretic and beta blocker groups, respectively, according to the type of blinding used. Regarding the use of ACE inhibitors or ARBs, significant improvement in the reduction of hospitalizations for heart failure or mortality from any cause could not be proven, highlighting the use of perindopril, quinapril, candesartan and irbesartan in the main studies used for this systematic review. However, the last one was found to be related to kidney function deterioration, which could increase the risk of cardiovascular death and hospitalization for heart failure. Likewise, use of these pharmacological groups together with loop diuretics or thiazides showed improved quality of life. Despite these findings, the hospitalization rates were similar, regardless of the type of diuretic.
In the study joining an ARB with a neprilysin inhibitor (sacubitril/valsartan), no significant differences were found in the rate of hospitalization for heart failure and death from cardiovascular causes. In addition, in the subgroup analysis, this medication suggested a benefit for patients with an ejection fraction between 45 and 57%, women (who made up a large proportion of the patients in the clinical trial), patients over the age of 65, and with concomitant mineralocorticoid receptor antagonists. On the other hand, impaired kidney function was found in groups in which valsartan alone was administered, as a comparator group. The use of aldosterone antagonist diuretics like spironolactone showed a lower number of hospitalizations for heart failure compared with placebo, with an increased risk of clinically irrelevant hyperkalemia at any time and a decreased glomerular filtration rate. Furthermore, a sub-analysis of this same group showed decreased mortality in women, who made up a significant proportion of the patients, which could suggest a benefit for females with this groups of medications. To date, ivabradine has not been associated with significant adverse events which would require treatment suspension.
For beta blocker use, we evaluated two studies, one with nebivolol and another with carvedilol, which showed no improvement in cardiovascular mortality and hospitalization.
Regarding SGLT2 inhibitors, statistical significance was found in the combined indicator of cardiovascular death and decreased hospitalization for heart failure. In addition, various sub-analyses showed a 5.8-point improvement in the quality-of-life score on the KCCQ scale, with a notably increased glomerular filtration rate, which resulted in a decreased use of diuretics. There was also a reduction in the number of hospitalizations, with a lower need for inotropes and fewer ICU admissions, highlighting the use of empagliflozin 10 mg/day and dapagliflozin 10 mg in patients with an LVEF >40% which significantly impacts the proposed outcomes.
According to the primary objectives proposed for this systematic review and the findings of the various studies evaluated, an improved quality of life was found with ACE inhibitor/ARB + diuretic treatment; a decreased hospitalization rate for heart failure with spironolactone, and a benefit with sacubitril/valsartan for patients with an LVEF between 45-57%, women and those over the age of 65. Recently, a reduction in the primary outcomes such as cardiovascular mortality, hospitalizations for pEF heart failure and increased glomerular filtration rate has been seen with the use of SGLT2 inhibitors, changes which previous studies had not been able to show. However, the adverse events reported, like hyperkalemia, hypotension, more genital and urinary tract infections and the risk of extremity losses do not constitute a contraindication, but rather an appropriate selection of patients, as the benefits outweigh the risks.
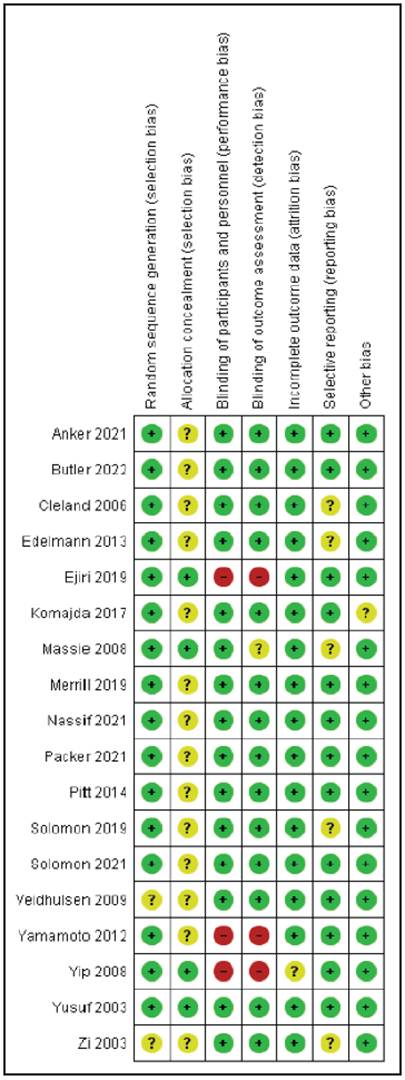
Figure 2 Bias risk assessment of the clinical studies which evaluated treatments for patients with pEF heart failure.
The result of this systematic review is comparable to Zheng et al.'s systematic review - meta-analysis, except for the findings related to the beta blocker group, in which they reported decreased mortality. However, it is relevant to note that these results are based on a single study by Aronow et al. in 1997, with a sample of 158 elderly patients with a prior myocardial infarction, 79 of whom were treated with propranolol, a result which, as previously suggested, differs from most of the published studies (Table 1).
Conclusions
The studies of SGLT2 inhibitors have shown a reduction in the combined risk of cardiovascular death, hospitalization for heart failure and improved quality of life according to the KCCQ scale. A therapeutic benefit was shown related to each patient's specific population characteristics. However, new avenues of study are needed, as the currently available treatment is not aimed at the various specific pathophysiological mechanisms of this heterogenous clinical syndrome.











 texto en
texto en