Introduction
In December 2019 in Wuhan, China, the first cases of patients with atypical pneumonia due to a new coronavirus (SARS-CoV-2) were found, and on April 10, 2020, the first case series of patients with SARS-CoV-2 neurological involvement was published 1. In the following months, the caseload of three neurological scenarios of the virus (unmasking, triggering and/or coexistent) increased based on the results of studies in animal models and published reports 2. SARS-CoV-2 has the S binding protein on its surface, with high affinity for the angiotensin 2 convert ing enzyme (ACE2) receptor, expressed in tissues such as respiratory tract epithelium, the kidney, small intestine and central nervous system, with special predilection for the motor and cingulate cortex, lateral ventricles, olfactory bulb and, more recently, discovered on the surface of neurons and glial cells 2,3. Trans-synaptic transfer from the periph eral nerve terminals (for example, in the respiratory tract) allows retrograde viral dissemination to the central (CNS) and peripheral nervous system (PNS) 4. Other proposed theories for CNS invasion have been through the olfactory bulb and blood-brain barrier (BBB) 5-7. Passage through the BBB may be due to leukocyte migration known as the "Trojan horse" mechanism and to direct passage through the pathologically permeable BBB as a result of the systemic inflammation characteristic of COVID-19 5-7. Now we are beginning to see the different neurological disorders, even in patients with neurological symptoms as the first sign of SARS-CoV-2 infection. We present a series of cases of hospitalized patients with diagnosed SARS-CoV-2 infection, through which we will review the evidence in the literature regarding neurological involvement.
Patients and methods
This was a retrospective observational study of a series of cases of SARS-CoV-2 infected patients with neurological abnormalities treated by the neurology service at Hospital Internacional de Colombia (HIC) from August 1 to December 31, 2020. The review included 30 patients of both sexes over the age of 18 with neurological abnormali ties on hospital admission or during their hospitalization, and a confirmed SARS-CoV-2 infection diagnosed by nasal swab and orolingual RT-PCR tests. The study adhered to the principles of the Declaration of Helsinki. This study was approved by the Research Ethics Committee (CEI in Span ish) at the Fundación Cardiovascular de Colombia-HIC.
All patients' electronic medical charts were reviewed along with their laboratory, CSF and radiological test findings. Information was gathered on age, sex, comorbidities, the National Institute of Health Stroke Scale (NIHSS), and typical COVID-19 symptoms (fever, cough, nasal congestion, diarrhea, sore throat, abdominal pain, nausea or vomiting), with which each patient was clas sified as either symptomatic or asymptomatic, using the American Thoracic Society 8 definition. The severity of COVID-19 and identification of the risk of requiring criti cal care was measured using the National Early Warning Score (NEWS2). Neurological symptoms were grouped according to the level of involvement as CNS (dizziness, headache, altered consciousness, acute neurovascular symptoms, aphasia, seizures, vertigo, disorientation) and PNS (anosmia, ageusia, visual disturbances, pain, muscle weakness). The radiological studies reviewed included chest x-rays and/or computed tomography (CT), simple head CTs and/or with contrast, simple brain magnetic resonance imaging (MRI) and/or with contrast, electro encephalogram reports, and other tests done according to each patient's clinical care needs.
Each of the neurological manifestations was confirmed by neurologists at the institution. Altered conscious ness was defined as a change in level of consciousness (somnolence, stupor and coma); confusion syndrome was defined as altered contents of consciousness (confusion and delirium); the diagnosis of a cerebrovascular accident (CVA) was confirmed through a head CT and/or brain MRI; seizures were diagnosed based on the clinical symptoms at the time of occurrence; inflammatory myelopathy was de fined as spinal cord involvement confirmed through spinal cord MRI; Guillain-Barré syndrome (GBS) was confirmed through nerve conduction studies; and posterior reversible encephalopathy syndrome (PRES) was confirmed by brain MRI. The functional outcome of patients with a CVA was measured using the modified Rankin Scale (mRS).
The information was stored in an Excel file previously coded by the investigators. The statistical analysis was performed using SPSS version 25; continuous variables were analyzed using averages with their respective standard deviation (SD) and categorical variables were expressed as absolute and relative frequencies, in percentages.
Results
Neurological involvement occurred in 30 patients with an average age of 65±17.3 years; 53% were male and 47% female. None of the patients were healthcare workers. Neurological involvement preceded the onset of respira tory symptoms and the diagnosis of SARS-CoV-2 infection (respiratory asymptomatic individuals) in 53% and was their reason for going to the hospital (Figure 1). In 47%, neurological involvement appeared during hospitalization for COVID-19, with a median of 5.5 days (Q1: 1-Q3: 36). Eighty-three percent had CNS involvement and 17% had PNS involvement. From a syndromic perspective, 43% (13) had a CVA, 10% (3) had seizures, 10% (3) had PRES, 7% (2) had encephalopathy, 7% (2) had a brief psychotic disorder, 3% (1) had myelopathy, 3% (1) had GBS, 3% (1) had a headache, 3% (1) had cerebral vasculitis and 3% (1) had an intracerebral hemorrhage. Only two patients had a reactivation and/or relapse of a preexisting neurological disorder, the first with a myasthenic crisis and the second with optic neuritis (Figure 1). Within their medical history, only 24% did not have any comorbidities, and 76% had one or more comorbidities, notably including hypertension, diabetes, obesity, cardiac arrhythmias and prior neurologi cal diseases.
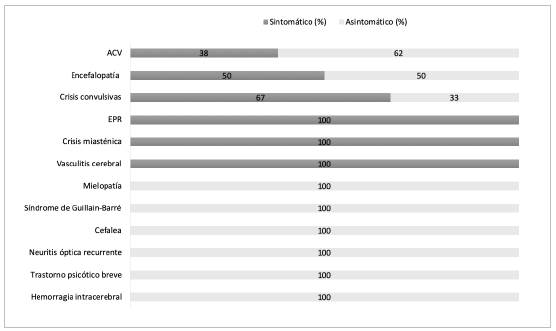
Figure 1 Distribution of the neurological abnormalities in patients with and without respiratory symptoms.
In eight out of the 13 patients with CVAs (61.53%), the onset of the cerebrovascular event preceded respiratory symptoms and SARS-CoV-2 infection diagnosis, while in the remaining five, the CVA was diagnosed within 24 hours after hospitalization for COVID-19 respiratory symptoms. The average age of these patients was 77±11.9 years. The chest x-ray and/or CT showed characteristic pulmonary lesions in all five patients with respiratory symptoms and in only three of the eight without respiratory symptoms on admission. All CVAs were large vessel CVAs, with 71.4% (10) in middle cerebral artery territory. The median NIHSS on admission of the respiratory asymptomatic patients was 15.5 (Q1: 6.75-Q3: 2.25). The NIHSS was only able to be assessed in two of the patients with respiratory symptoms (three and five points, respectively), as the remaining patients were under sedation in the intensive care unit (ICU) due to ventilatory problems. The NEWS 2 scale had a median of three points (Q1: 1-Q3: 6). The mRS at discharge was 0-2 in 38%, 3-4 in 16%, and six patients (46%) died (three from respiratory problems and three from intraparenchymal hemorrhagic transformation).
Three patients (61, 69 and 79 years old) with no history of epilepsy had seizures. One of the patients was admitted for generalized tonic-clonic seizures (six episodes in 15 days) with no prior respiratory symptoms. The two remain ing patients had had ageusia, anosmia and mild respiratory symptoms in the one to eight days prior. In all cases, the chest x-ray showed typical bilateral alveolar opacities. In two of the cases, the electroencephalogram showed diffuse slowing without epileptiform activity. The head CT was normal in only one patient, in another case it showed diffuse cerebral edema and in the third it showed right frontal subcortical bleeding. Two of the patients died from COVID-19 acute adult respiratory distress syndrome (ARDS).
The three patients diagnosed with PRES, aged 45, 54 and 58, had had to be admitted to the ICU due to their respiratory problems. Neurological symptoms were evident in two pa tients during the first 48 hours after extubation, characterized by persistent altered consciousness in one case and a seizure in another. These two patients died from COVID-19 ARDS. In the third patient, neurological involvement began one month after the onset of COVID-19, with left hemiparesis, global aphasia and cortical blindness. This patient recovered completely from the neurological involvement three days later. A brain MRI in the three patients showed signs of sym metrical bilateral vasogenic edema involving the parietal and occipital juxtacortical white matter (Figure 2C).
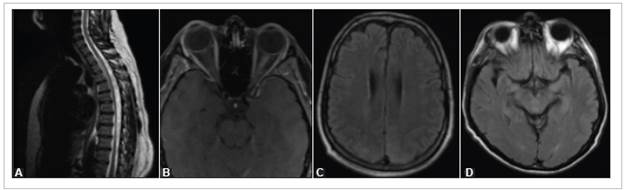
Figure 2 Lesions in patients in the series. A) Patient 10: T2-weighted MRI of the dorsal spinal column showing hyperintensity of the spinal cord due to longitudinally extensive myelopathy. B) Patient 17: T1-weighted orbital MRI with fat saturation showing thickening and enhancement of the left optic nerve. C) Patient 26: Brain MRI with slight bilateral, symmetrical cortical-subcortical hyperintensity in the parietal-occipital region on T2-FLAIR sequences. D) Patient 29: Brain MRI with hyperintensity in both hippocampi and the central and posterior mesencephalon in T2-FLAIR sequences.
Two patients, aged 58 and 76, were diagnosed with acute encephalitis. They both had a normal head CT. One of them was admitted with hypertensive encephalopathy without respiratory symptoms, and the second had respiratory symp toms requiring ventilatory support and developed signs and symptoms compatible with encephalopathy (somnolence and myocloni) on day four. In both cases, the chest x-ray showed typical lung opacities, and the outcome was toward improve ment, the first after two months and the second after 30 days.
A 34-year-old respiratory asymptomatic patient debuted with acute neuropathic pain (dermatomes T4-T6), right lower limb paresis, left lower limb paralysis and abnormal sphincter control. On physical exam, she had lower limb hyperreflexia and spasticity. The chest x-ray was normal, and the spinal cord MRI showed hyperintensity and contrast medium enhancement from the first to the fifth segment of the dorsal spinal cord (Figure 2A). Demyelinating lesions in other parts of the CNS were ruled out and the patient improved significantly after five days' treatment with methylprednisolone.
A 71 -year-old patient was diagnosed with Guillain-Barré syndrome after debuting with three days of rapidly ascend ing flaccid areflexic quadriparesis and facial diparesis. This patient was admitted with no respiratory symptoms and his chest x-ray on admission was normal. Eight days after the onset of symptoms, his CSF showed albuminocytologic dissociation (proteins: 148.1 mg/dL), and nerve conduction tests were compatible with mixed axonal and demyelinating sensorimotor polyneuropathy, with the absence of F waves in all four extremities. The patient received five plasma pheresis exchanges with significant recovery of strength and movement in all four extremities.
A 27-year-old patient developed a moderate oppressive occipital headache six days prior to being admitted to the emergency room, which rapidly became severe. He also had phonophobia, proximal myalgias and fatigue, but no fever, cough, abdominal pain or diarrhea. The neurological exam, chest x-ray and head CT were normal. Intravenous ibuprofen did not improve his symptoms. He was diagnosed with SARS-CoV-2 infection on his second day in the hos pital. After seven days on intravenous steroids, the patient improved. He was discharged with instructions to complete his quarantine and follow outpatient treatment.
A 65-year-old patient presented to the emergency room complaining of eight days of a severe, oppressive bilateral frontal headache, general malaise, fever, runny nose and arthralgia. A chest x-ray showed lung opacities. On his first day of hospitalization, he was diagnosed with SARS-CoV-2 infection. Four days later his headache worsened, and he experienced fleeting amaurosis in his left eye. The CSF analysis was completely normal (FilmArray, Genexpert, KOH, India ink, Gram and adenosine deaminase were negative). The T2-FLAIR brain MRI showed hyperintensity in both hippocampi and in the central and posterior regions of the mesencephalon (Figure 2D), diagnosing cerebral vasculitis. He was admitted to the neurological ICU and given acetaminophen and intravenous fluids. His symptoms improved 18 days later, and he was discharged with no symptoms.
Two patients were admitted to the emergency room due to motor restlessness, irritability, and structured visual hallucinations, and were diagnosed with brief psychotic disorder (DSM V). The first patient, a 59-year-old with respiratory symptoms, had hypoglycorrhachia (39 mg/dL) and an infectious etiology was ruled out (KOH, India ink, Gram, adenosine deaminase, GenExpert and FilmArray were negative). His chest x-ray and head CT were normal; this patient was treated by psychiatry with risperidone and was discharge on day 10 due to clinical improvement. The second case was a 74-year-old man with no respiratory symptoms. His chest x-ray showed pulmonary lesions, and his chest CT had signs of bilateral pulmonary thromboembolism. He died on the third day of hospitalization due to respiratory complications.
A 52-year-old patient with a history of myasthenia gravis was admitted with acute ventilatory failure as part of a myasthenic crisis. A tomography and chest x-ray on admission showed typical COVID-19 changes and right pulmonary thromboembolism, requiring orotracheal intu bation for nine days. He was treated with five plasmapheresis exchanges, pyridostigmine and methylprednisolone, progressed favorably, and was successfully extubated and discharged to home.
A 23-year-old patient with a prior diagnosis of idiopathic optic neuritis of the left eye was admitted for a two-day his tory of decreased visual acuity in her right eye.
This patient had no respiratory symptoms and her chest x-ray on admission was normal. An orbital and spinal cord MRI showed thickening and hyperintensity of the intraorbital, intracanalicular and intracranial portions of the left optic nerve with marked contrast medium enhancement and thickening of the intracranial portion of the right optic nerve up to the optic chiasm, with no spinal cord involvement (Figure 2B). She was treated with methylprednisolone (1 g/day for three days), recovered her visual acuity, and was discharged on outpatient steroids.
Overall mortality was 35% (11), 50% (7) in patients ad mitted with respiratory symptoms and 25% (4) in patients who consulted for neurological symptoms only (respiratory asymptomatic individuals). The cause of death was identified as ARDS in five patients, intracerebral hemorrhage in three, and intracranial hypertension in three.
Discussion
Our study found that neurological abnormalities were the first clinical manifestation in 53% of the patients, with no respiratory symptoms, and SARS-CoV-2 was diagnosed as part of the general screening protocols during the pandemic. This finding is a key warning in clinical practice because although a time relationship cannot be established in some cases, SARS-CoV-2 infection can debut as a neurological syndrome, with no respiratory symptoms, and even be the only sign of the infection (Figure 3) 9. Although neuro logical COVID-19 is less common than pulmonary disease, inpatient case series have reported that in 13.5% of the patients, neurological symptoms occur two days after the onset of the initial respiratory symptoms, in 43% they occur simultaneously and, contrary to what we found in our cases, in only 2% are neurological symptoms the COVID-19 debut 10. The most frequent CNS abnormalities were CVAs, seizures, encephalopathy, PRES and parainfectious compli cations like longitudinal myelitis, GBS, optic neuritis and vasculitis, similar to what has been published to date 11.
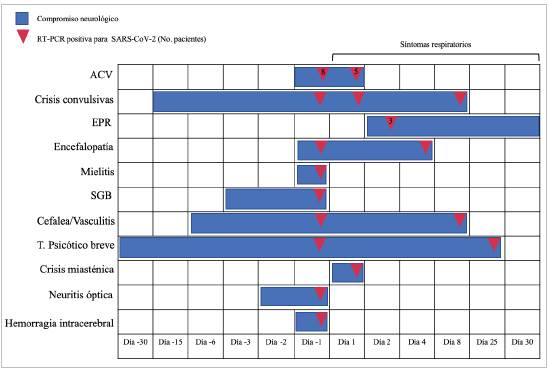
Figure 3 Chronology of neurological involvement from the onset of the disease and days from the onset of respiratory symptoms to SARS-CoV-2 diagnosis. Day one corresponds to the day on which respiratory symptoms began. (In color).
During the SARS-CoV-2 pandemic, more severe CVAs with greater inpatient mortality have been reported in young adults with no comorbidities and large vessel involvement, and in older adults with cardiovascular comorbidities (12 -17). In most patients in our series, the CVA was the first sign, as they were respiratory asymptomatic individuals, similar to what has been published recently suggesting that the risk of CVA is greater in respiratory asymptomatic young adults, even months after COVD-19 diagnosis 18. In inpatient CVA cases, the diagnosis was early (a mean of one day), unlike what has been reported in the literature (a mean of 10 days) 1. In only one patient was a defined cardioembolic etiology determined, corresponding to atrial fibrillation 4, with the caveat that etiological studies could not be performed in six patients due to their rapid deteriora tion and death. Autopsies on COVID-19 patients have shown lesions very suggestive of CNS (among other organ) isch emia, which could be related to the associated prothrombotic and hypercoagulable state in COVID-19 19-21. Now, the causal relationship between SARS-CoV-2 and CVA is still a matter of debate, but the fact is that this viral infection is associated with severe and highly fatal CVAs, conditioned by the respiratory involvement in COVID-19 and, in others, the severity of the CVA as well 22.
A reduced seizure threshold has been associated with viral infections 23,24, and in COVID-19, the cytokine storm has been proposed as a possible cause of seizures and altered consciousness in patients with a history of epi lepsy 1,25. None of the three patients in our series had a history of epilepsy, and in the two cases with seizures and respiratory involvement, the outcome was death. It is esti mated that 10% of SARS-CoV-2 positive patients who are intubated and sedated in the ICU have subclinical seizures (25), which were only found in one of our cases who was diagnosed with PRES, a disease with a greater incidence in patients with severe COVID-19 and ICU admission, older age, underlying comorbidities and high mortality 25,26.
As occurs in other viral infections, SARS-CoV-2 may predispose to the development of peripheral nerve diseases like GBS and neuromuscular junction diseases like myasthenia gravis 27. Guillain-Barré syndrome in patients with COVID-19 presents between days 10 and 21 after the onset of respiratory symptoms, with a predominance of the variants with cranial nerve involvement (47%) 27. The recommended treatment continues to be immunoglobulin or plasmapheresis, as these do not affect the natural immu nity 27. On the other hand, immunosuppression has been proposed as protective in reducing the cytokine storm, for example in patients with myasthenia gravis who, although they experience more severe COVID-19, tend to have a positive outcome, as seen in patient 14 in our series 28.
Neurological complications in patients with SARS-CoV-2 infection may be direct, according to the ACE2 receptor nerve expression, such as headaches, anosmia and dysgeusia, symptoms which are more frequent for days or between the seventh and tenth day after the onset of COVID-19 29,30. According to the intensity or severity, headache in and of itself is not one of the most frequent chief complaints in the emergency room of tertiary care hospitals, which would explain the low incidence of this symptom in a hospital series 30. There may also be indirect complications as a result of hypoxia and parainfectious disorders, due to the host's adaptive immune response (Table 1) 31. The parainfectious phenomenon has been described in cases of acute necrotizing myelitis associated with axonal polyneuropathy and optic neuritis with positive anti-MOG antibodies, which respond rapidly to the use of corticosteroids 32-35, as seen in our patient with optic neuritis. Postinfectious complications, for the most part, cover a wide spectrum of neuropsychiatric manifestations like depression, anxiety, and mood disorders, as well as the neurocognitive-behavioral sphere including memory problems and psychotic disorders 36, as seen in our patients 9 and 19.
Table 1 Classification of neurological complications according to the neuroinvasive nature of SARS-CoV-2.
| Indirect | Direct | Parainfectious |
|---|---|---|
| CVA | Anosmia | Acute disseminated encephalomyelitis |
| Headache | Hyposmia | Myelitis |
| Ataxia | Dysgeusia | Optic neuritis |
| Acute necrotizing | Myasthenia gravis | |
| encephalopathy | ||
| Altered consciousness | Guillain-Barré syndrome | |
| Seizures | Parkinsonism |
Although there is no expert consensus, in this pandemic, regardless of the respiratory involvement in patients with neurological symptoms, at the very least, a chest x-ray should be ordered. Also, a simple head CT as the initial imaging test in stable patients with a clinical suspicion of CVA, and a brain and/or spinal MRI in cases where medical decisions depend on it or when spinal cord and spinal root assessment are needed 37.
The authors of this article recognize its limitations, begin ning with its sample size. However, a number of cases are presented with a general hospital base in which most showed symptoms of neurological involvement before SARS-CoV-2 respiratory involvement was evident, and information is contributed on the abnormalities caused by this virus, with a different behavior than that found in other publications. In addition, it should be clarified that patient enrollment was done prior to beginning the national vaccination plan against SARS-CoV-2, which occurred in April 2021.
Conclusions
Neurological involvement due to SARS-Co-V-2 causes a wide variety of symptoms, with CVAs being the most frequent. In more than half of our patients, neurological abnormalities presented as the first manifestation. Mortality in patients with neurological manifestations is associated with respiratory complications and is higher in patients with CVAs.











 texto em
texto em 


