Introduction
Malignant hypercalcemia (MH) is the most common electrolyte disorder in adults with cancer, with a prevalence ranging from 3 to 30% 1,2. It occurs most frequently in advanced stages of the disease, and in 80% the patho-physiological mechanism is parathyroid hormone-related protein activation 1,3-5. Its frequency varies according to the type of cancer, and different studies report squamous cell carcinoma (SCC) to be the most frequent (30-62%), principally lung cancer (35%), followed by breast cancer (25%), and hematological cancers (14%), especially multiple myeloma 6-8.
Due to its potential neurological, renal and cardiovascu lar involvement, it merits prompt study and treatment. Its correction limits life-threatening complications and allows cancer treatment to continue 2,9-11. Treatment of MH is focused on hydration and the use of biphosphonates, as well as denosumab in refractory cases or those with kidney failure 1,2,9,12-14. In general, MH marks a poor prog nosis with an 18-22% in-hospital mortality rate, and mean survival ranging from 30 to 50 days 2,15-19.
Although it has been found more frequently in patients with certain types of cancer and some of the risk factors related to greater mortality have been described, includ ing solid organ disease, male sex, secondary neurological involvement and onset more than 140 days after cancer diagnosis 2,12,15,18,20, there is no information show ing an association with factors like age, sex, kidney disease, nutritional status, or immobility, among others, which could modify the risk of MH.
The clinical behavior of MH, its associated cancer di agnoses, hospital stay, and the risk factors associated with its presentation in the Colombian cancer population are unknown. This study focused on obtaining this information, which would be very useful for clinical management and to generate measures to control the risk factors associated with its onset. To achieve this objective, patients treated at the Instituto Nacional de Cancerología (INC), a Colombian national referral center, were studied retrospectively.
Materials and methods
Population
Research subjects with solid or hematological-lymphoid tumors and MH who consulted in the INC emergency room or hospital wards from 2014-2019.
Cases
Research subjects over the age of 18 with a confirmed diagnosis of MH (albumin-corrected serum calcium >10.5 mg/dL, excluding non-cancer causes of hypercalcemia). A total of 230 research subjects were collected and analyzed in the predetermined time period.
Controls
Research subjects over the age of 18 with some type of histologically confirmed neoplasm, with a normal albumin-corrected serum calcium measurement (8.5-10.4 mg/dL) in the predetermined time period. Simple random sampling of the eligible subjects was conducted, including those who met the inclusion criteria and did not meet the exclusion criteria. A total of 223 controls were selected. The variable for pair ing cases and controls was the proportion of subjects with SCC, for which a 5% or lower difference between groups was established. This was selected as the pairing variable as it is the main and most consistent determining variable for the onset of MH and its outcomes, allowing the impact of other variables on the onset of MH to be evaluated, and controlling selection bias.
Exclusion criteria of cases and controls
Pregnant women, those with missing data for more than 20% of the variables, and those with a < 48-hour observa tion period in the INC emergency room or hospital wards were excluded.
Analysis
The database was exported from the RedCap system to the statistical software as disidentified data, and data were analyzed as a group. Univariate analysis was conducted, with categorical variables described with relative and absolute frequencies. Numerical variables were described accord ing to their nature as averages and standard deviation or as medians and interquartile range. A bivariate analysis was conducted to explore possible associations between the evaluated variables and the onset of MH, using Fisher's test or Chi2 for categorical variables and the Student's t or Mann-Whitney U test for numerical variables, according to their distribution. Variables with a statistically significant association (p < 0.05) were included in the non-predictive explanatory logistic regression model. In addition, a minimum of 10 observations per variable included in the model were established a priori to avoid bias due to an insufficient sample size. In the event that not all variables were able to be included due to a lack of observations, those with greater statistical association on the bivariate analysis were selected.
Ethical considerations
This study was conducted in line with the Colombian ethical considerations according to Resolution No. 008430 of 1993, which establishes the scientific, technical and administrative norms for health research, ensuring adher ence to the principles of beneficence and non-maleficence, autonomy and justice. The study was approved by the Uni versidad Nacional de Colombia Ethics Committee and the INC Ethics Committee.
Results
The study groups were similar in terms of the age and sex variables. The Karnofsky index in the case group had a median of 60% (IQR 50%-80%) and the control group had a median of 80% (IQR 60-90%) (p < 0.00001). The average albumin level in the case group was 2.8 g/dL (SD 0.72) vs. 3.5 g/dL (SD 0.70) in the control group (p < 0.00001). The median creatinine level in the case group was 1.09 mg/dL (IQR 0.77-1.76) and 0.87 mg/dL (0.62-1.3) in the control group, (p = 0.0001). The median initial corrected calcium in the cases was 12.98 mg/dL (IQR 11.64-14.42) and 9.4 mg/dL (IQR 9.02-9.74) in the controls. Regarding the histopathological characteristics, the proportion of SCC was 34.34% in the cases and 28.69% in the controls (Table 1). The distribution of cancers associated with MH is shown in Figure 1, with a clear predominance of cervical SCC (16.52%), breast cancer (13.04%), multiple myeloma (10%) and lymphoma (8.69%).
Table 1 Demographic variables of cases and controls.
| Variable | Cases (n=230) | Controls (n=223) |
|---|---|---|
| Age, median (IQR), years | 59 (50-68) | 59 (48-68) |
| Male sex No. (%) | 95 (41.30) | 81 (36.32) |
| Squamous cell carcinoma No. (%) | 79 (34.34) | 64 (28.69) |
| Karnofsky index, median (IQR), % | 60 (50-80) | 80 (60-90) |
| Albumin, mean (SD), g/dL | 2.87 (0.72) | 3.5 (0.70) |
| Initial corrected calcium, median (IQR), mg/dL | 12.98 (11.64-14.42) | 9.4 (9.02-9.74) |
| Creatinine, median (IQR), mg/dL | 1.09 (0.77-1.76) | 0.87 (0.62-1.30) |
| Presence of metastasis No. (%) | 138 (60.00) | 89 (39.91) |
| Bone metastasis No. (%) | 65 (47.1) | 21 (23.59) |
| Active cancer treatment No. (%) | 109 (47.39) | 123 (55.16) |
| Chemotherapy No. (%) | 75 (68.80) | 93 (75.60) |
| Surgery No. (%) | 23 (21.10) | 30 (24.39) |
| Radiation therapy No. (%) | 25 (22.93) | 23 (18.69) |
| Hospital stay, median (IQR), days | 16 (9-27) | 9(6-17) |
| In-hospital death No. (%) | 112 (48.70) | 37 (16.59) |
| SD: standard deviation, IQR: interquartile range, n: frequency |
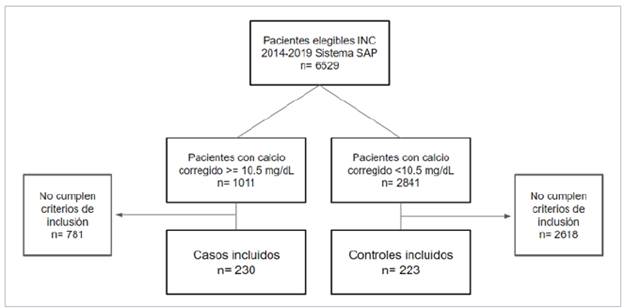
Figure 1 shows the patients included in the study after applying the inclusion and exclusion criteria.
The median number of days of hospital stay was 16 for cases and nine for controls, and in-hospital death showed a marked difference: 48.70% of the cases vs. 16.59% of the controls.
The proportion of metastases was greater in the cases with 60 vs. 39.91% in the controls. Fifty low or normal PTH values were reported in the cases.
One case of primary hyperparathyroidism associated with parathyroid carcinoma was found, with a PTH level of 1,474 pg/mL. Seventy percent of the cases had clinical signs and symptoms of hypercalcemia. The most common were neurological, in 54.34% of the cases, while 13.91% had gas trointestinal manifestations, and 3.04% had cardiovascular manifestations. Regarding the treatment of hypercalcemia cases, 99.56% received intravenous hydration with normal saline solution, and 46.08% also received a biphosphonate (86.79% zoledronic acid and 13.2% ibandronic acid). The median initial corrected calcium was 12.98 mg/dL; at 72 hours it was 12.27 md/dL, and between 72 hours and seven days of follow up it was10.72 mg/dL. Resolution of the ini tial clinical manifestations was seen during inpatient follow up in 41.61% of the patients (Table 2).
Table 2 Descriptive follow up variables in the malignant hypercalcemia group.
| Variable | Cases (n=230) | |
|---|---|---|
| Initial corrected calcium, median (IQR), mg/dL | 12.98 (11.66-14.42) | |
| Corrected calcium < 72 hours, median (IQR), mg/dL | 12.27 (11.1-13.31) | |
| Corrected calcium from 72 hours - 7 days, median (IQR), mg/dL | 10.72 (9.88-12.02) | |
| Neurological manifestations No. (%) | 125 (54.34) | |
| Cardiovascular manifestations No. (%) | 7 (3.04) | |
| Gastrointestinal manifestations No. (%) | 32 (13.91) | |
| Treatment with hydration No. (%) | 229 (99.56) | |
| Treatment with biphosphonates No. (%) | 106 (46.08) | |
| Zoledronic acid No. (%) | 92 (86.79) | |
| Ibandronic acid No. (%) | 14 (13.2) | |
| Resolution of the clinical manifestations No. (%) | 67 (41.61) | |
| IQR: interquartile range, n: frequency |
Bivariate analysis
The proportion of SCC was similar (p = 0.196), which allowed pairing by this variable, as proposed. There was a statistically significant difference in in-hospital mortality between cases and controls (p < 0.0001). The descriptive analysis of in-hospital mortality in the group of patients who died showed a lower Karnofsky index, with a median of 60% (IQR 50-80); a higher initial corrected calcium, with a median of 12.0 mg/dL (IQR 10.53-14.01) vs. 9.78 mg/dL (IQR 9.21-12.03); lower albumin levels, with an average of 2.79 g/dL vs. 3.32 g/dL; greater frequency of metastasis (63.76 vs. 43.42%) and greater frequency of neurological manifestations (74.11 vs. 35.59%) (Table 3).
Table 3 Descriptive analysis of in-hospital mortality
| Variable | In-hospital death | ||
|---|---|---|---|
| Yes | No | ||
| Cases No. (%) | 112 (48.7) | 118 (51.3) | |
| Controls No. (%) | 37 (16.59) | 186 (83.41) | |
| Age, median (IQR), years | 62 (51-69) | 59 (49-68) | |
| Male sex No. (%) | 61 (40.94) | 115 (37.83) | |
| Female sex No. (%) | 88 (59.06) | 189 (62.17) | |
| Squamous cell carcinoma No. (%) | 44 (29.53) | 99 (32.57) | |
| Karnofsky index, median (IQR), % | 60 (50-80) | 70 (60-90) | |
| Albumin, mean (SD), g/dL | 2.79 (0.76) | 3.32 (0.70) | |
| Initial corrected calcium, median (IQR), mg/dL | 12.0 (10.53-14.01) | 9.78 (9.21-12.03) | |
| Creatinine, median (IQR), mg/dL | 1.0 (0.77-1.80) | 0.91 (0.64-1.4) | |
| Presence of metastasis No. (%) | 95 (63.76) | 132 (43.42) | |
| Bone metastasis No. (%) | 34 (22.82) | 52 (17.11) | |
| Non-bone metastasis No. (%) | 61 (40.94) | 80 (26.32) | |
| Active cancer treatment No. (%) | 71 (47.65) | 161 (52.96) | |
| Neurological manifestations No. (%) | 83 (66.40) | 42 (35.59) | |
| SD: standard deviation, IQR: interquartile range, n: frequency | |||
Multivariate analysis
The albumin, Karnofsky index, creatinine, metastasis and type of metastasis variables were included (p < 0.05). The type of metastasis was excluded from the logistic regression analysis since it is subrogated to the presence of metastasis and the number of observations was smaller than the total group of cases and controls.
For the logistic regression analysis, variables showing an association with the presence or absence of MH were included: normal albumin values OR 0.41 (95% CI 0.29 0.55), Karnofsky index (greater than or equal to 70%) OR 0.98 (95% CI 0.97-0.99) and presence of metastasis OR 1.87 (95% CI 1.23-2.84) (Table 4).
Table 4 Multivariate analysis.
| Variable | Crude OR | Adjusted OR | ||
|---|---|---|---|---|
| P value | OR (95% CI) | P value | ||
| Albumin | 0.34 (0.25-0.46) | < 0.0001 | 0.41 (0.29-0.55) | < 0.0001 |
| Karnofsky index | 0.97 (0.96-98) | < 0.0001 | 0.98 (0.97-0.99) | 0.001 |
| Metastasis | 2.25 (1.52-3.34) | < 0.0001 | 1.87 (1.23-2.84) | 0.003 |
| Age, years | 1.0 (0.98-1.01) | 0.895 | ||
| Male sex | 1.23 (0.82-1.83) | 0.276 | ||
| Creatinine | 1.03 (0.92-1.15) | 0.558 | ||
| Active cancer treatment | 0.73 (0.49-1.07) | 0.09 | ||
| Squamouos cell carcinoma | 1.29 (0.85-1.97) | 0.196 | ||
| OR: odds ratio, CI: confidence interval. | ||||
Discussion
Malignant hypercalcemia is an oncological emergency which affects patients' quality of life and short-term mortal ity 21-23.
In the patients who developed MH, the predominant his tological type was SCC, consistent with what is described in the literature 1,3,24. In our case, this was principally cer vical SCC, breast adenocarcinoma, multiple myeloma and lymphomas. In the United States, the three most common types of cancer in MH are lung cancer, multiple myeloma and breast cancer 25; in the United Kingdom they are lung cancer, multiple myeloma and kidney cancer 6,16; and in Brazil they are head and neck, lung and breast tumors 24. Gynecologic cancer is described as an unusual cause of MH; however, in other studies, it was mainly associated with vulvar and cervical SCC 26-28. These differences may be due to INC patients predominantly having skin, gastrointestinal, breast and cervical cancer, with a lower prevalence of lung cancer and hematologic tumors 29. This finding suggests the need to strengthen and improve prevention, screening and education strategies for cervical cancer, developing public health policies to provide access to vaccination and timely diagnosis to avoid progression to advanced stages.
In the United States, 45% of the patients with MH have metastasis 25. In our study, 60% had metastasis, with 28.26% having bone metastasis, in line with what is re ported internationally (26-46%) 8,16,24,30. Non-bone metastasis was more common in patients who died, with 40.93%. This finding may be related to a greater proportion of patients with advanced disease being treated at INC, as this is a national referral center.
A smaller proportion of the patients in our study were on active cancer treatment, compared with the global reports (70 vs. 47.6%), possibly related to the fact that a large proportion were receiving palliative care 25,31.
In a study of patients with MH (Ramos RE, et al.), the average initial calcium value was greater than 12 mg/dL, and 55% of the patients with MH had altered mental status, similar to the findings in our study 24. On the other hand, 25% of the patients in a survival analysis had neurological signs and symptoms, 92% improved their calcium levels with hydration, biphosphonates and diuretics, and 8% died 8. In our study, this group of patients had a 66.4% in-hospital mortality rate, much higher than what was previously described, which could be related to the higher frequency of advanced stages of cancer.
Although the use of biphosphonates has not been shown to decrease mortality, symptom improvement has a signifi cant impact on cancer patients' quality of life. In a study of MH prevalence, 31% of the patients received biphosphonates or denosumab, a finding similar to what was reported in our study 32-35. The most commonly used biphosphonate was zoledronic acid (86.79%), followed by ibandronic acid (13.2%). In patients with filtration rates lower than 30 ml/ min/1.72 m2, ibandronic acid was used, due to its lower risk of nephrotoxicity 33-37, and denosumab was reserved as a second line medication for those refractory to the initial treatment.
Hypercalcemia in the general population is associated with a 6% mortality rate, with an HR of 1.88 (1.184; 2989, P = 0.007) 38; in patients with MH, 30-day mortality is more than 50%, similar to what we found in our study (48.69%) 8,15,31,37,39.
The median length of hospital stay in our study was greater than that of other publications (16 days vs. four days) 16,26,40.
This is the first study in Colombia evaluating the clinical and paraclinical factors associated with MH which, when identified early, can be addressed to change the natural course of the disease. A Karnofsky index > 70% and normal albumin were found to be protective factors and the presence of metastasis was a risk factor.
For example, diagnosing cancer and optimizing the nu tritional status early could decrease the risk of MH, leading to a better quality of life and decreased hospital stay 41.
The limitations of this study include the fact that PTH val ues were not available for all MH cases, to provide a better description of the disease, and precise electrocardiographic records could not be obtained to adequately characterize the cardiovascular manifestations.











 text in
text in