Introduction
Cerebral amyloid angiopathy (CAA) accounts for 15% of all non-traumatic intracerebral hemorrhages in people over the age of 60 1. In older adults (OAs) over the age of 70, it represents 50% of all lobar hematomas 2. It is characterized by extracellular deposits of fibril proteins (β-amyloid) in the walls of small and medium vessels of the cerebral cortex and meninges, mainly affecting the parietal and occipital lobes 3. It is a significant cause of lobar intracerebral hemorrhages and cognitive impairment in OAs and is associated with a high prevalence of small vessel disease markers, including white matter hyperintensities and cerebral microhemorrhages 4. In patients with Alzheimer's disease, the prevalence of CAA is 48% 5, and in the general population the prevalence is 23% 5.
Amyloid deposits in the vessel media may be extensive enough to cause vessel wall necrosis, microaneurysm forma tion and lobar hemorrhaging 4. These patients may present with a broad clinical spectrum including cognitive impair ment, lobar intracerebral hemorrhage and transient focal neurological episodes 6. The most common clinical mani festation of CAA is acute lobar intracerebral hemorrhage; these hemorrhages can cause hemiparesis due to effects on the motor neurons and pyramidal tracts. A large lobar hem orrhage can cause decreased consciousness through a direct effect or secondary mass effect on the activating reticular system networks 7.
Although CAA occurs to a certain degree in almost all cases of Alzheimer's disease, it may occur independently, especially in the elderly 8. It can also occur sporadically, or with familial occurrence; for example, Icelandic hereditary cerebral hemorrhage with amyloidosis (HCHWA-I), and hereditary cystatin C amyloid angiopathy 9.
All the complications of CAA are determinant for the onset of geriatric syndromes and conditions in the OA population, with a greater risk of catastrophic disability, progressive func tional decline, rapidly progressive neurocognitive impairment, chronic immobility syndrome, a high risk of pressure ulcers, dysphagia and malnutrition, as well as high mortality rates 4.
The importance of this case presentation lies in the fact that this clinical condition is almost exclusive to OAs, often unsuspected and has negative health consequences, not only in decreasing life expectancy, but also affecting the quality of life and producing high rates of disability, dependency and social isolation.
We present the case of an OA who consulted for a de novo seizure episode and neurological focalization, in whom lobar intracerebral hemorrhages were found at two different times during the clinical course. These findings were attributed to CAA, with an adverse clinical course ending in her death.
Case presentation
An 80-year-old woman consulted due to a three-day insidious holocranial headache and subsequent decreased state of consciousness coupled with de novo generalized tonic-clonic seizures, with subsequent neurological focalization, sphincter incontinence, disorientation and dysarthria.
She had a history of systemic arterial hypertension treated with amlodipine 5 mg/day and losartan 50 mg/12 hours; she denied diabetes, the use of recreational drugs, smoking or a relevant family history.
Prior to the current clinical condition, she was indepen dent in her activities of daily living, with no complaints of memory loss and no nutritional or neurological impairment.
On her admission physical exam, she had a blood pressure of 110/70 mm Hg, a pulse of 89 bpm, and a RR of 18 bpm; she was afebrile and had a pulse oximetry of 97% on room air. Her cardiopulmonary exam was normal. Neurologically, she was somnolent but arousable, disori ented to time and place, oriented to person, and had left hemiparesis without sensory impairment; cranial nerve assessment showed central facial paralysis, her tendon reflexes were normal, her gait could not be assessed, and she had a Glasgow score of 12/15.
The relevant admission laboratory tests showed a normal coagulation time and platelet count. Admission brain imag ing (computed tomography, magnetic resonance imaging with contrast, angiography and magnetic resonance angiography) showed an acute, multifocal macrohemorrhage of the right brain, perilesional edema and ipsilateral subarachnoid hemorrhage, in addition to signs of mild brain hypotrophy and changes raising the suspicion of chronic microangiopa thy (Figure 1). She had no radiological signs of intracranial lesions like tumors, vascular malformations, aneurysms, or arterial and/or venous stenosis or occlusion.
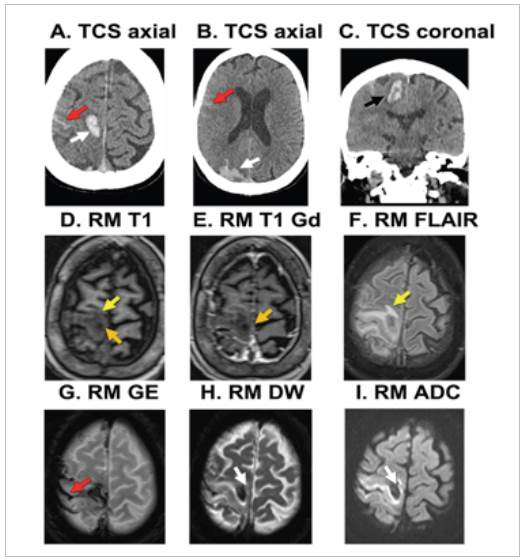
Figure 1 Initial brain images. A-C: non-contrast computed tomography (NCCT); D -1: magnetic resonance imaging (MRI). Acute right parieto occipital and precentral circumvolution bleeds (white arrows) affecting the cortical, subcortical and deep white matter regions (black arrow); acute Fisher II right parieto-temporal subarachnoid hemorrhage (red arrow) and right brain edema, manifested in the asymmetric size of the cerebral sulci and changes in the adjacent perilesional tissue (yellow arrow). There is no enhancement with the contrast medium (orange arrow).
The case was interpreted as possible CAA, medical treat ment was provided with pharmacological blood pressure control and basic support measures like adequate hydration and anti-seizure treatment. Neurosurgical management was not considered because the hemorrhage was not causing a mass effect or signs of intracranial hypertension.
During her hospital stay, the patient continued to have left hemiparesis and somnolence. On day 11 of her stay, the patient's neurological status deteriorated further, and she became comatose. A follow up simple brain tomography showed a new left macrohemorrhage with similar character istics to the one already described, draining to the ipsilateral ventricle (Figure 2). At that point, basic support and comfort measures were offered, with no invasive measures or inten sive care unit admission criteria due to her poor neurological prognosis. The patient died on her 15th day in the hospital.
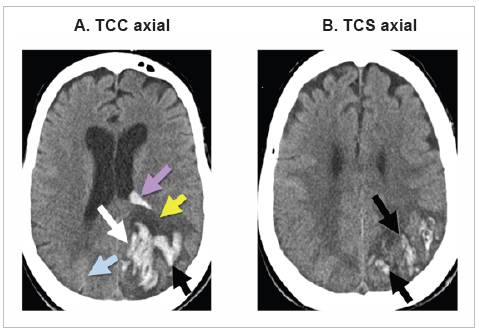
Figure 2 Non-contrast computed tomography (NCCT) images on day 11 of hospitalization. A new acute left parieto-occipital bleed is seen (white arrow) also affecting the cortical, subcortical and deep white matter regions (black arrow). Note the greater severity of the bleed andperilesional edema (yellow arrow), with partial compression of the occipital horn of the ipsilateral ventricle and internal bleeding (purple arrow). The right parieto-occipital bleed is not very noticeable now, due to its age (blue arrow); compare withFigure 1B.
Discussion
The presented patient was an 80-year-old OA, in whom cases of CAA are more frequent, constituting a significant cause of lobar intracerebral hemorrhages in this group of people 2,10. The incidence depends largely on age, be ing more common in those over the age of 80. In a series of 784 autopsies, the prevalence of CAA ranged from 2.3% for patients 65 to 74 years old, to 8.0% for those 75 to 84 years old, and 12.1% for patients over the age of 85 11.
In OAs with CAA, the most common clinical manifesta tion is acute lobar intracerebral hemorrhage, which occurs more frequently in the posterior regions of the brain, with neurological symptoms depending on the affected site 11. When the hemorrhagic lesions are small, they may be associated with focal deficits or transient focal neurologi cal symptoms attributed to dysfunction of the underlying cortex. Our patient debuted with a seizure, altered state of consciousness and subsequent neurological focalization, with macrohemorrhages found on the cerebral tomography (Figure 1).
The presence of CAA should be considered as a dif ferential diagnosis in OA patients with acute or chronic hemorrhage findings on magnetic resonance imaging of the brain without an obvious alternative cause. In this patient's case, she was found to have a history of HTN, but was being treated and had well-controlled blood pressure on admission to the hospital. In addition, the magnetic resonance findings showed a superficial lobar hemorrhage characteristic of CAA and there were no small areas of deep hemorrhages, which are more frequently found in hypertensive hemorrhaging, an important distinctive finding which therefore suggests that the etiology of cerebral hemorrhaging in this patient was related more to CAA than to a hypertensive emergency 1.
Definitive CAA is only diagnosed post-mortem 12. A complete pathological exam of the brain showing amyloid deposits with vasculopathy, evidence of cerebral hemorrhaging and a lack of other diagnostic lesions, confirms the diagnosis. The Boston criteria were proposed in 1990 to standardize the definition of CAA including clinical, imaging and pathological criteria 12. According to these criteria, probable CAA is diagnosed in patients over the age of 55 with an appropriate clinical history and magnetic resonance findings showing multiple hemorrhages of varying sizes and ages restricted to the lobar, cortical, and cortical-subcortical region (cerebellar lesions are allowed), with no other ex planation. In the case presented, the patient fulfilled all the criteria, which supported the diagnosis of probable CAA aided by the MRI, in which two or more lesions in the lobar regions of the brain, not attributable to other causes 12, should be sought. In this case, the patient had two areas of cerebral macrohemorrhaging located in different territories and at different times in the clinical case progression, with no other clear cause of non-traumatic cerebral hemorrhaging, which is highly suggestive of CAA.
The onset of subcortical intracerebral hemorrhaging located in different sites and/or on separate occasions in a non-hypertensive OA patient, especially in the presence of dementia, suggests hemorrhaging related to cerebral amyloid angiopathy 13. However, hypertension may be present in these cases and dementia is not an absolute prerequisite, as seen in this patient, who had a history of well-controlled hypertension and did not have hypertensive crises on admis sion or during the course of the condition. She had no major neurocognitive disorder, either, as there was no complaint of prior memory problems or other cognitive disorders in the context of a patient who was completely independent in her activities of daily living.
Hematomas found on computerized tomography are important in the differential diagnosis 13. Lobar macro-hemorrhages were found in this patient, located in different territories and at different times during the course of the condition (Figures 1 and 2), with a brain vessel angiography ruling out vascular lesions or other etiologies which could explain the origin of these hemorrhages.
In another vein, surgery for non-life-threatening CAA-related cerebral hemorrhaging is controversial 13. By the same token, surgical intervention is the only way to obtain a reliable positive diagnosis, although clinical data and brain tomography tracking can suggest it, especially if there are several repetitive bleeding episodes, as in the presented case. Surgery also involves an inherent risk of deterioration due to re-bleeding, and therefore it is not recommended solely for obtaining a histopathological diagnosis, except under special circumstances such as when there is a possible dif ferential diagnosis or if the patient explicitly wants it 13. Otherwise, conservative management with close monitoring seems to be the better alternative 2. There is no medical treatment for CAA; in this patient's case, medical manage ment was provided using conventional support measures, with a special emphasis on blood pressure control, adequate hydration and the prevention of further seizures.
An unfavorable prognosis in the event of lobar hemorrhaging is associated with advanced age (over the age of 80) and the size of the hematoma 14. Overall mortality for acute lobar hemorrhaging due to CAA ranges from 10 to 30%, with a better prognosis for patients with smaller hematomas (< 50 ml) and higher levels of consciousness on admission (Glasgow Coma Scale ≥ 8) 14. The factors associated with this patient's death included the size of the hematoma, advanced age, and the presence of multiple le sions at different points in the course of the clinical condi tion, which led to her decline and demise.
The functional trajectory leading to the patient's death can be explained using the compression of morbidity theory 15, in which a catastrophic illness (in this case, CAA) occurs at the end of life, but prior to this event, the patient was able to have a healthy life with adequate functioning up to a very advanced age, and she only lost her autonomy at the end of her functional trajectory. Therefore, her period of disability was very short; only at the end of her life did she experience a highly deteriorated health status, severe functional impairment, high healthcare costs and lack of definitive treatment, ultimately resulting in all the outcomes which occurred 15.











 texto em
texto em 


