Introduction
A new virus emerged in December 2019, in Wuhan, China, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an RNA virus similar to SARS-CoV and MERS-CoV. This virus is the etiological agent of COVID-19, named by the World Health Organization (WHO) and declared a pandemic in 2020 1-4. In most cases, the disease is mild, with upper respiratory symptoms, general malaise and usually self-limited fever 4. However, in 15% of cases it may be severe, with pneumonia requiring hospitalization, and up to 5% may have a severe disease with organ dysfunction, respiratory failure and the need for intensive care, with mortality ranging from 1.4 to 5% 4-6. The infection occurs after close contact with patients or asymptomatic carriers, mainly through droplets, aerosols, or direct or surface contact 4,7. Through January 2022, COVID-19 has caused 306 million cases and 5.4 million deaths worldwide 8. In Colombia, 5.3 million cases and 130,000 deaths have been confirmed.
The S protein expressed by SARS-CoV-2 mediates viral entry to host cells, binding to angiotensin-converting enzyme 2 (ACE2). Various cells in the human body express ACE2, for example, respiratory, digestive, renal, cardiac, hematological and neurological system cells 10, making them susceptible to infection 2,11. The gastrointestinal system has a high expression of ACE2 in esophageal mucosa cells, ileal and colon enterocytes, and liver cells, which allows SARS-CoV-2 to infect these cells and cause a variety of gastrointestinal symptoms 2,10,12. Anorexia has been reported in up to 98% of cases, diarrhea in up to 49% with an average duration of four days and three daily episodes, nausea and vomiting in 10%, abdominal pain in 9% and positive fecal occult blood or leukocytes in 7% 3,5,10,12,13. A meta-analysis with more than 4,000 Asian patients with COVID-19 showed a positive fecal RT-PCR in 50% of the patients, and gastrointestinal symptoms in almost 20%. In addition, patients with severe COVID-19 had more gastrointestinal symptoms than those with non-severe cases (17.1 vs. 11.8%) 14. SARS-CoV-2 can cause liver damage due to both direct infection, secondary to ACE2 expression in the cholangiocytes and hepatocytes, as well as due to cytokine storm, ischemic hepatitis, or medication toxicity 3,15,16. Elevated transaminases have been found in up to 53% of patients, elevated bilirubins in 17%, and elevated alkaline phosphatase in 2% 3,17.
There is insufficient data in Colombia on the prevalence and characteristics of gastrointestinal involvement in patients with COVID-19. This study seeks to determine the prevalence of gastrointestinal involvement in patients with COVID-19 treated at two hospitals in a healthcare sub-network in Bogotá (Colombia), both on admission and during hospitalization, as well as to determine the association between COVID-19 gastrointestinal involvement and hospital stay, severity and mortality from COVID-19.
Objectives
The primary objective is to describe the prevalence of gastrointestinal involvement in patients with COVID-19 treated at two hospitals in a sub-network of hospitals in Bogotá (Colombia). The secondary objectives are to determine the prevalence of diarrhea, bloody diarrhea anorexia, anosmia, dysgeusia, dysphagia, odynophagia, vomiting, gastrointestinal bleeding with its main causes, abdominal pain, and abnormal liver profiles in patients with COVID-19 in the study population, both on admission as well as during the hospital stay; determine the association between COVID-19 gastrointestinal involvement and hospital stay as well as COVID-19 severity and mortality; and describe the endoscopic findings and diagnostic and therapeutic endoscopic procedures performed on patients with COVID-19 and gastrointestinal involvement.
Materials and methods
This was a cross-sectional study carried out within a hospital sub-network in Bogotá (Colombia) from February 2020 to March 2021. Patients over the age of 18 with a SARS-CoV-2 infection diagnosis confirmed by respiratory RT-PCR were included. Patients with an inaccessible or incomplete medical chart or one with significant inconsistencies, and referred or pregnant patients, were excluded. COVID-19 was classified as critical when there was invasive mechanical ventilation, shock or multiple organ dysfunction; severe if the oxygen saturation was <90%, the respiratory rate was >30 breaths per minute, and there were pulmonary infiltrates in >50% of the lung fields; moderate if there was pulmonary involvement with dyspnea or chest x-ray abnormalities with an oxygen saturation >90%; and mild if there was no dyspnea or hypoxia and it was managed at home 18. Approval was obtained from the ethics and research committee of the hospital network where the study was performed and from the ethics committee of the Universidad Nacional de Colombia.
Statistical analysis
A sample size of 246 patients was calculated using the Pan American Health Organization's EPIDAT version 3.1 software, with a 20% estimated prevalence of gastrointestinal involvement in patients with COVID-19, a 95% confidence level, and a 5% alpha error.
A Microsoft Excel® table was constructed for data analysis, which was subsequently purged and analyzed using IBM’s SPSS® statistical software.
The statistical analysis used frequencies and percentages for categorical qualitative variables. Quantitative variables are presented using measures of central tendency (mean and standard deviation). The Chi2 test and p value were used to determine the differences in the liver profile.
Prevalence was expressed in proportions with 95% confidence intervals (CIs), and the association between gastrointestinal involvement and the clinical outcomes was expressed as odds ratios (ORs) with 95% confidence intervals. A multivariate model was constructed based on a regression analysis between the variables with statistical significance and the outcomes of interest.
Results
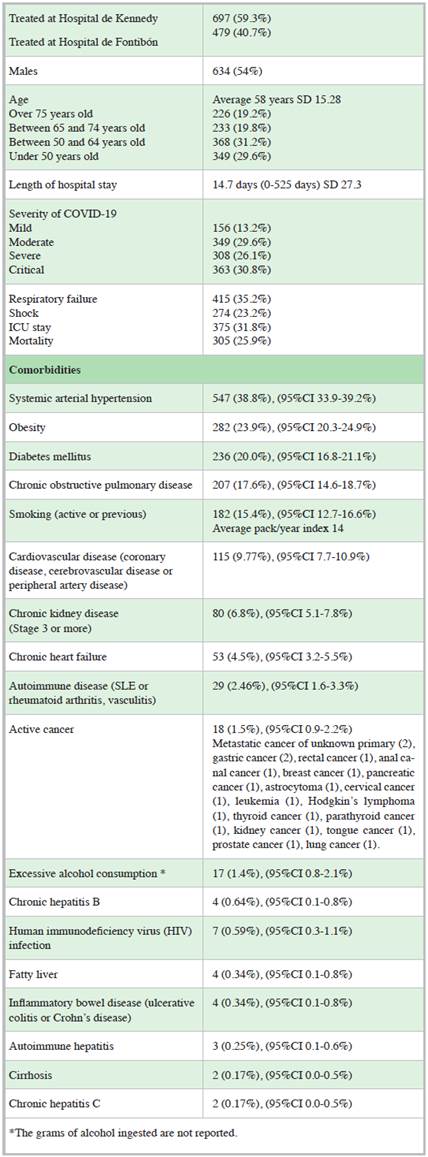
A total of 1,715 patients over the age of 18 with COVID-19 confirmed by RT-PCR were evaluated for the study (900 patients at the first hospital and 815 patients at the second hospital). Altogether, 539 patients were excluded (398 had incomplete medical charts, inconsistencies in the medical charts, uncertain COVID-19 diagnoses or inaccessible medical charts, 133 were referred, and eight were pregnant), for a total of 1,176 patients included in the study. Fifty-four percent were males, and the average age was 58 years. Table 1 shows the baseline characteristics of the included patients.
The prevalence of gastrointestinal manifestations in the study population was 50% (95%CI 47-52%). The most common gastrointestinal manifestations were diarrhea (18.4% 95%CI 15.3-19.5% and 7.14% 95%CI 5.4-8.2%, on admission and during hospitalization, respectively), odynophagia on admission (17.6% 95%CI 14.6-18.8%) and anorexia on admission (14.7% 95%CI 12.0-15.8%). Table 2 shows the characteristics of gastrointestinal involvement both on admission as well as during hospitalization.
Table 2 Gastrointestinal manifestations of COVID-19 on admission and during hospitalization.
| On admission | During hospitalization | |
|---|---|---|
| Prevalence of gastrointestinal manifestations: 50% (95%CI 47-52%) | ||
| Diarrhea | 217 (18.4%), (95%CI 15.3-19.5%) Average duration: five days SD 5.29 | 84 (7.14%), (95%CI 5.4-8.2%) Average duration: 2.4 days SD 2.27 |
| Bloody diarrhea | 6 (0.51%), (95%CI 0.2-1.0%) | 1 (0.08%), (95%CI 0.0-0.4%) |
| Anorexia | 173 (14.7%), (95%CI 12.0-15.8%) | N/A |
| Anosmia | 115 (9.77%), (95%CI 7.7-10.9%) | N/A |
| Dysgeusia | 84 (7.14%), (95%CI 5.4-8.2%) | N/A |
| Dysphagia | 30 (2.55%), (95%CI 1.7-3.4%) | N/A |
| Odynophagia | 208 (17.6%), (95%CI 14.6-18.8%) | N/A |
| Vomiting | 84 (7.14%), (95%CI 5.4-8.2%) | 35 (2.97%) |
| Gastrointestinal bleeding | 17 (1.44%), (95%CI 0.8-2.1%) (melena 8, hematemesis 1, hematochezia 1, rectorrhagia 1) | 42 (3.57%) (melena 20, hematemesis 5, hematochezia 3, rectorrhagia 2) |
| Abdominal pain | 104 (8.84%), (95%CI 6.9-9.9%) | 65 (5.52%) |
The multivariate analysis showed an association between the onset of diarrhea during hospitalization and a prolonged hospital stay (>14 days) (OR of 1.93, 95%CI 1.19-3.13). Gastrointestinal bleeding, whether on admission or during hospitalization, was associated with mortality, respiratory failure, ICU admission, prolonged hospital stay, and a critical disease course. No statistically significant association was found between anosmia, dysgeusia or abdominal pain and worse clinical outcomes. Table 3 shows the association between gastrointestinal involvement and clinical outcomes.
Table 3 Gastrointestinal manifestations and clinical outcomes in COVID-19 (OR with 95%CI) (multivariate analysis).
| Mortality | Respiratory failure | Shock | Need for ICU | Prolonged hospital stay | Critical COVID-19 | |
|---|---|---|---|---|---|---|
| Anosmia | 0.65 (0.35-1.19) | 0.66 (0.38-1.12) | 1.48 (0.85-2.55) | 1.06 (0.63-1.78) | 0.86(0.50-1.46) | 0.81 (0.47-1.4) |
| Dysgeusia | 0.79 (0.39-1.6) | 1.08 (0.59-1.96) | 0.81 (0.42-1.57) | 0.78 (0.42-1.43) | 0.91 (0.49-1.68) | 0.90 (0.48-1.67) |
| Diarrhea on admission Diarrhea during hospitalization | 0.94 (0.57-1.22) 0.67 (0.37-1.21) | 1.01 (0.72-1.42) 0.98(0.59-1.61) | 1.07 (0.73-1.57) 1.18 (0.68-2.03) | 1.12 (0.79-1.58) 1.2 (0.74-1.99) | 0.73 (0.51-1.05) 1.93 (1.19-3.13) | 0.99 (0.7-1.42) 0.87 (0.51-1.48) |
| Vomiting on admission Vomiting during hospitalization | 0.53 (0.27-1.01) 0.92 (0.41-2.1) | 0.51 (0.28-0.91) 2.41 (1.19-4.88) | 0.53 (0.27-1.03) 3.54 (1.74-14.82) | 0.55 (0.31-0.99) 3.05 (1.50-6.19) | 0.85 (0.49-1.46) 2.66 (1.32-5.36) | 0.52 (0.28-0.97) 2.14 (1.05-4.34) |
| Gastrointestinal bleeding on admission Gastrointestinal bleeding during hospitalization | 3.13 (1.1-9.1) 3.3 (1.77-6.15) | 2.8 (1.01-8.11) 3.1 (1.7-8.88) | 5.14 (1.78-14.8) 9.1 (4.6-18.07) | 2.31 (0.81-6.57) 9.11 (4.6-18.07) | 2.79 (1.01-7.74) 3.65 (1.93-6.9) | 3.02 (1.04-8.75) |
| Abdominal pain on admission Abdominal pain during hospitalization | 0.63 (0.35-1.14) 1.18 (0.62-2.26) | 0.42 (0.24-0.74) 1.49 (0.82-2.7) | 0.48 (0.25-0.92) 1.22 (0.63-2.37) | 0.46 (0.26-0.81) 1.59 (0.88-2.89) | 0.70 (0.42-1.19) 1.48 (0.83-2.63) | 0.36 (0.19-0.66) 1.38 (0.74-2.56) |
Thirty-four patients (2.8%) underwent upper gastrointestinal endoscopy. The main findings were chronic gastritis (32 patients) and erosive gastritis (12 patients), documenting four patients with gastric ulcers, three Forrest III ulcers and two Forrest lib ulcers, one of which required adrenaline injection. Esophageal varices were ligated in a 67-year-old male patient with cirrhosis, critical COVID-19 and gastrointestinal bleeding; unfortunately, the patient died. Eleven patients (1%) underwent colonoscopy, with the most frequent findings being internal hemorrhoids 8 and diverticulosis 5. Endoscopic retrograde cholangiopancreotography (ERCP) was performed on three patients (only two reports were available) and gastrostomy on 10 patients (0.85%). The endoscopic procedures performed are found in Table 4.
Table 4 Endoscopic procedures performed on COVID-19 patients.
Within the study population, 11 patients (0.93%) had acute pancreatitis. According to the Atlanta classification, four had severe pancreatitis, four moderately severe and three mild. Only 2.2% of all patients underwent any intra-abdominal diagnostic imaging on admission, and none had bile duct dilation. A total of 3.5% of the patients underwent some form of intra-abdominal diagnostic imaging during hospitalization and, of these, four had bile duct dilation.
Liver profile abnormalities occurred in 46% of the study patients (95%CI 43-49%). The most common abnormality was elevated transaminases, with ALT ≥ 45 U/L in 30.1% (95%CI 27-32%) on admission and in 40.2% (95%CI 2743%) during hospitalization, and AST ≥1 45 U/L in 36.2% (95%CI 33-39%) on admission and in 42.6% (95%CI 39-45%) during hospitalization. Elevated total bilirubin ≥ 1.5 mg/dL was found in 2.89% (95% CI 1.9-3.9%) on admission and 8.7% (95% CI 7.2-10.4%) during hospitalization. Table 5 shows the liver profile abnormalities in the study population. In general, the transaminase, bilirubin and alkaline phosphatase abnormalities were mild. Table 6 shows the differences in transaminase, bilirubin and alkaline phosphatase levels according to the severity of COVID-19, using the Chi2 test to determine the p value. Statistically significant differences were found in the three tests, with higher values as the severity of the disease increased or death occurred.
Table 5 Liver profile abnormalities in COVID-19 patients.
| On admission1 | During hospitalization2 | |
|---|---|---|
| Total bilirubin | Average: 0.77 mg/dL, | Average: 1.68 mg/dL, |
| SD 2.0 mg/dL | SD 10 mg/dL | |
| <1.5 mg/dL | 876 (74.7%) | 727 (62.0%) |
| ≥ 1.5 - 2.9 mg/dL | 26 (2.2%) | 74 (6.3%) |
| ≥3.0 -5.9 mg/dL | 2 (0.1%) | 16 (1.3%) |
| >6.0 mg/dL | 7 (0.6%) | 13 (1.1%) |
| No information | 266 (22.6%) | 347 (29.6%) |
| Alanine aminotransferase ALT | Average 69 IU/L, | Average 101 IU/L, |
| SD 343 IU/L | SD 245 IU/L | |
| <45 IU/L | 594 (50.6%) | 376 (32.0%) |
| ≥45 - 89 IU/L | 261 (22.2%) | 266 (22.6%) |
| ≥90 -299IU/L | 97 (8.2%) | 184 (15.6%) |
| >300 IU/L | 13 (1.1%) | 36 (3.0%) |
| No information | 212 (18.0%) | 315 (26.8%) |
| Aspartate aminotransferase AST | Average 67 IU/L, | Average: 108 IU/L, |
| SD 279 IU/L | SD 306 IU/L | |
| <45 IU/L | 522 (44.5%) | 349 (29.7%) |
| ≥45 - 89 IU/L | 342 (29.1%) | 292 (24.9%) |
| ≥90 -299 IU/L | 91 (7.7%) | 185 (15.7%) |
| >300 IU/L | 11 (0.9%) | 38 (3.2%) |
| No information | 211 (18.0%) | 313 (26.7%) |
| Alkaline phosphatase | Average 118 IU/L, | Average: 142 IU/L, |
| SD 87 IU/L | SD 127 IU/L | |
| <200IU/L | 285 (24.3%) | 317 (27.0%) |
| ≥200 - 599 IU/L | 30 (2.5%) | 65 (5.5%) |
| ≥600 IU/L | 3 (0.2%) | 5 (0.4%) |
| No information | 859 (73.2%) | 790 (67.4%) |
| Albumin | Average 2.94 g/dL, | N/A |
| SD 0.5 g/dL | ||
| ≥3.5 g/dL | 34 (2.9%) | |
| ≥2.5 -3.4 g/dL | 97 (8.2%) | |
| <2.5 g/dL | 35 (2.9%) | |
| No information | 1,011 (86.2%) | |
| Prothrombin time | Average: 13.0 s, | Average: 14.0 s, |
| SD 2.7 s | SD 5.0 s | |
| <14 s | 183 (15.6%) | 220 (18.7%) |
| ≥14-16s | 36 (3.0%) | 65 (5.5%) |
| ≥17-29 s | 12 (1.0%) | 26 (2.2%) |
| ≥30 s | 2 (0.2%) | 7 (0.6%) |
| No information | 944 (80.5%) | 859 (73.2%) |
1 Taken within 48 hours of admission. 2 Highest value during hospitalization.
Table 6 Liver profile, COVID-19 severity and mortality.
| Critical % of N subtables | COVID-19 severity | Mortality | Pearson's Chi-square test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | YES | NO | |||||
| % of N subtables | % of N subtables | % of N subtables | Count | Count | P | ||||
| ALT on admission | Not reported | 4.0% | 7.9% | 10.0% | 4.8% | 60 | 254 | 26.145 | .001*bc |
| <45 U/L | 1.9% | 0.4% | 0.8% | 13 | 23 | ||||
| 46-90 U/L | 8.3% | 0.3% | 3.1% | 4.0% | 68 | 116 | |||
| 91-300 U/L | 8.7% | 1.4% | 5.5% | 7.1% | 76 | 190 | |||
| >300 U/L 8.0% | 3.7% | 10.6% | 9.6% | 88 | 288 | ||||
| AST on admission | Not reported | 2.1% | 7.3% | 4.5% | 3.9% | 32 | 178 | 48.154 | .000*bc |
| <45 U/L | 0.7% | 0.1% | 0.2% | 7 | 4 | ||||
| 46-90 U/L | 3.4% | 0.2% | 2.1% | 2.0% | 41 | 50 | |||
| 91-300 U/L | 11.2% | 1.3% | 7.9% | 8.7% | 104 | 238 | |||
| >300 U/L | 13.4% | 4.5% 15.0% 11.4% | 121 | 401 | |||||
| AST during hospitalization | Not reported | 3.8% | 7.9% | 10.0% | 4.8% | 58 | 254 | 83.859 | .000*bc |
| <45 U/L | 2.5% | 0.4% | 0.3% | 21 | 17 | ||||
| 46-90 U/L | 9.1% | 0.1% | 2.2% | 4.3% | 85 | 100 | |||
| 91-300 U/L | 9.3% | 1.3% | 6.7% | 7.5% | 83 | 209 | |||
| >300 U/L | 6.1% | 4.0% | 10.3% | 9.3% | 58 | 291 | |||
| Total bilirubin on admission | Not reported | 3.3% | 8.1% | 6.6% | 4.5% | 42 | 223 | 22.757 | .004*bc |
| <1.5 md/dl | 0.3% | 0.2% | 0.2% | 3 | 4 | ||||
| 1.6-3.0 mg/dL | 0.1% | 0.1% | 1 | 1 | |||||
| 3.1-6.0 mg/dL | 1.0% | 0.1% | 0.5% | 0.6% | 8 | 18 | |||
| >6.0 mg/dL | 26.2% | 4.9% | 22.5% | 20.8% | 251 | 625 | |||
| Total bilirubin during hospitalization | Not reported | 3.8% | 8.3% | 11.6% | 5.7% | 60 | 286 | 51.499 | .000*bc |
| <1.5 mg/dl | 0.6% | 0.2% | 0.3% | 8 | 5 | ||||
| 1.6-3.0 mg/dL | 1.2% | 0.2% | 8 | 8 | |||||
| 3.1-6.0 mg/dL | 4.2% | 0.1% | 0.6% | 1.4% | 37 | 37 | |||
| >6.0 mg/dL | 21.0% | 4.7% | 17.5% | 18.6% | 192 | 535 | |||
| Alkaline phosphatase on admission | Not reported | 13.3% | 12.0% | 23.0% | 18.8% | 166 | 623 | 38.102 | .000*bc |
| < 200 U/L | 0.3% | 0.1% | 1 | 4 | |||||
| > 200-600 U/L | 3.9% | 0.3% | 0.5% | 0.8% | 32 | 33 | |||
| >600 U/L | 13.3% | 0.8% | 6.1% | 6.6% | 106 | 211 | |||
A statistically significant association was found between elevated transaminases and respiratory failure, shock, prolonged hospital stay, a critical disease course and mortality, with a tendency toward a stronger association at higher transaminase levels (Table 7). Severely elevated alkaline phosphate levels (≥ 600 U/L) were associated with mortality (OR 2.32 95%CI 1.09-4.95). An important limitation was the high percentage of patients whose albumin (86.2%) and prothrombin time (PT) (80.5%) were not measured; however, it was enough to show a statistically significant association between hypoalbuminemia and poor outcomes. For example, albumin <2.5 g/dL on admission was associated with mortality (OR 8.9 95%CI 2.9-27.5). No significant association was found between PT abnormalities and clinical outcomes.
Table 7 Liver profile abnormalities and clinical outcomes in patients with COVID-19 (data expressed in ORs with 95%CI).
| Mortality (OR) | Respiratory failure | Shock | Prolonged hospital stay 1 | Critical COVID-191 | |
|---|---|---|---|---|---|
| ALT on admission <45 IU/L ≥45 - 89 IU/L ≥90 -299 IU/L >300 IU/L | 0.95 (0.68-1.31) 0.86 (0.53-1.4) 2.9 (0.96-8.78) | 1.22 (0.91-1.64) 1.04 (0.68-1.63) 1.89 (0.63-5.7) | 1.13 (0.81-1.56) 1.16 (0.72-1.86) 3.33 (1.1-10.07) | 1.13 (0.84-1.54) 0.9 (0.57-1.43) 1.65 (0.55-4.97) | 1.21 (0.89-1.64) 1.02 (0.65-1.61) 2.3 (0.77-6.98) |
| ALT during hospitalization <45 IU/L ≥45 - 89 IU/L ≥90 -299 IU/L >300 IU/L | 1.3 (0.92-1.87) 1.91 (1.3-2.8) 1.84 (0.9-3.8) | 1.94 (1.4-2.7) 3.41 (2.36-4.9) 3.95 (1.94-8) | 2.31 (1.57-3.4) 4.5 (3-6.7) 9.7 (4.65-20.23) | 2.25 (1.61-3.16) 2.41 (1.67-3.5) 3.6 (1.79-7.21) | 1.8 (1.32-2.62) 3.41 (2.36-4.95) 4.71 (2.32-9.6) |
| AST on admission <45 IU/L ≥45 - 89 IU/L ≥90 -299 IU/L >300 IU/L | 1.45 (1.06-1.96) 2.71 (1.71-4.3) 5.8 (1.67-20.1) | 1.62 (1.23-2.16) 2.48 (1.58-3.89) 5.4 (1.42-20.71) | 1.48 (1.08-2.02) 2.65 (1.67-4.22) 9.4 (2.46-36.15) | 1.19 (0.9-1.6) 1.34 (0.85-2.12) 1.17 (0.34-4) | 1.44 (1.09-1.92) 1.8 (1.15-2.85) 6.14 (1.6-23.4) |
| AST during hospitalization <45 IU/L ≥45 - 89 IU/L >90 -299 IU/L >300 IU/L | 1.99 (1.36-2.9) 4.3 (2.84-6.4) 6.19 (3.1-12.5) | 2.48 (1.77-3.5) 6.61 (4.46-9.8) 11.01 (5-24.2) | 3.01 (1.96-4.6) 10.5 (6.77-16.57) 23.6 (10.6-52.4) | 1.59 (1.14-2.22) 2.7 (1.86-3.92) 4.1 (2.1-8.2) | 2.32 (1.63-3.3) 5.3 (3.6-7.79) 12.3 (5.6-27.35) |
| Total bilirubin on admission <1.5 mg/dL ≥1.5 - 2.9 mg/dL ≥3.0 -5.9 mg/dL >6.0 mg/dL | 0.75 (0.03-16.6) 1.69 (0.3-9.09) 1.88 (0.42-8.33) | 1.33 (0.06-9.1) 1.35 (0.25-7.14) 2 (0.45-9.21) | 1.33 (0.06-31.12) 0.4 (0.07-2.3) 0.51 (0.11-2.27) | 1 (0.8-1.2) 1.14 (0.16-4.7) 2.5 (0.55-11.11) | 0.75 (0.03-16.06) 0.88 (0.16-4.7) 0.63 (0.03-6.25) |
| Total bilirubin during hospitalization <1.5 mg/dL ≥1.5 - 2.9 mg/dL ≥3.0 -5.9 mg/dL >6.0 mg/dL | 1.6 (0.36-7.14) 1.6 (0.47-5.55) 1.22 (0.38-1.43) | 0.15 (0.01-1.54) 0.833 (0.25-3) 3.7 (1.15-12.5) | 0.27 (0.03-1.8) 1.25 (0.25-4) 4.8 (1.72-16.67) | 1.03 (0.2-4.8) 1.22 (0.34-4.34) 4.54 (1.36-14.28) | 0.16 (0.-03-1.36) 0.56 (0.01-2) 2.27 (0.33-1.32) |
| Alkaline phosphatase on admission <200 IU/L ≥200 - 599 IU/L ≥600 IU/L | 2.32 (1.09-4.95) | 3.45 (0.28-42.6) 1.7 (0.15-18.97) | 2 (0.16-24.5) 1.06 (0.095-11.88) | 1 (0.08-12) 1.45 (0.13-16.22) | 1.42 (0.66-3) |
| Alkaline phosphatase during hospitalization <200 IU/L ≥200 - 599 IU/L ≥600 IU/L | 3.8 (0.4-36.6) 2.01 (0.22-18.2) | 1.3 (0.16-2.24) 0.26 (0.03-2.4) | 8.4 (0.88-79.6) 2.4 (0.27-22.35) | 1.21 (0.19-7.8) 1.8 (0.03-3.9) | 1.31 (0.21-5.71) 4.1 (0.18-3.6) |
| Albumin on admission ≥3.5 g/dL ≥2.5 -3.4 g/dL <2.5 g/dL | 3.13 (1.2-8.3) 8.9 (2.9-27.5) | 3.7 (1.61-8.5) 12.5 (3.8-41.17) | 3.07 (1.3-7.27) 9.37 (3.13-28.07) | 2.42 (1.1-5.37) 3.57 (1.31-9.7) | 3.63 (1.53-8.6) 11.11 (3.6-34.2) |
| PT on admission <14 s ≥14-16 s ≥17-29 s >30 s | 2 (0.1-41) 2.1 (0.04-9.3) 1.7 (0.03-12.5) | 3 (0.14-64) 3.5 (0.19-62) 1.41 (0.09-22.8) | 2 (0.09-41) 1.11 (0.06-19.3) 1.1 (0.14-6.7) | 2 (0.024-10.25) 2.27 (0.13-39.7) 1.1 (0.07-17.9) | 2.99 (0.14-64.2) 2.27 (0.13-39.7) 1.2 (0.07-19.55) |
| PT during hospitalization <14 s ≥14-16 s ≥17-29 s ≥30 s | 1.02 (0.19-5.5) 1.34 (0.08-3.93) 2.27 (0.5-11.11) | 1.08 (0.17-6.9) 1.77 (0.31-10.22) 1.33 (0.03-3.35) | 1.35 (0.0364-3.87) 4 (0.22-6.25) 6.66 (0.03-10.2) | 3.35 (0.038-3.64) 3.33 (0.04-2.9) 4 (0.022-1.6) | 2.1 (0.054-7.7) 1.8 (0.067-5.5) 4.87 (0.26-7.65) |
| 1 Longer-than-average hospital stay (14 days). 2 Critical COVID-19: Respiratory failure with mechanical ventilation, shock or multiple organ dysfunction | |||||
Discussion
This study included more than 1,000 patients with COVID-19 confirmed by RT-PCR in respiratory samples, which is, to the authors' knowledge, the study with the most patients published in our country on the gastrointestinal manifestations of this disease. The severity of COVID-19 in the study population was critical in 30.8%, severe in 26.1%, moderate in 29.6% and mild in 13.2%, with respiratory failure in 35.2%, the need for ICU in 31.8% and a mortality of 25.9%, which is higher than reported in other studies 4,19,20. However, this could be due to this study's setting (the emergency rooms of secondary and tertiary care institutions). It should be noted that understanding the gastrointestinal manifestations of the disease has greater clinical significance in patients with moderate and severe COVID-19, given their risk of a critical course, complications and death.
This study included patients from two different hospitals within the same hospital network, providing the benefits of a multicenter study at different levels of complexity. The most frequently reported comorbidities in the study population were systemic arterial hypertension (38.8%), obesity (23.9%), diabetes mellitus (20%), chronic obstructive pulmonary disease (17.6%) and smoking (15.4%), which is similar to what is reported in other studies and series of patients with COVID-19 20,21. The prevalence of gastrointestinal diseases was less than 1% for various diseases (gastrointestinal cancer, inflammatory bowel disease, cirrhosis, chronic hepatitis B, chronic hepatitis C, autoimmune hepatitis and fatty liver), with a possible underreporting of some entities such as fatty liver.
The prevalence of gastrointestinal manifestations in the study population (50%) is within the range reported in other studies 3,10,13,20. In a meta-analysis, the prevalence of gastrointestinal involvement in COVID-19 patients was 20% 14 and in another meta-analysis it was 15%; with, however, a range of 2-57% and high heterogeneity (I 2=96%) 22. The most common gastrointestinal manifestations on hospital admission were diarrhea (18.4%) with an average duration of five days, anorexia (14.7%), odynophagia (17.6%) and abdominal pain (8.8%). These data are similar to what has been found in studies of other populations 3,5,10,13. Gastrointestinal involvement in COVID-19 is related to SARS-CoV-2's ability to infect various cells in the gastrointestinal tract due to the presence of ACE2 receptors which bind to the viral S protein 2,10,23. COVID-19 patient care in our country does not recommend fecal RT-PCR for disease diagnosis or surveillance 24. However, studies in other countries have shown the presence of SARS-CoV-2 in the stool even after negativization of the RT-PCR in respiratory samples 14. Dysgeusia and ansomia, which have been described as symptoms suggestive of COVID-19 4, were found in 7.14 and 9.77%, respectively. These symptoms were not associated with poor outcomes in the study population.
The multivariate analysis found a statistically significant association between some gastrointestinal manifestations and poor clinical outcomes in patients with COVID-19. Diarrhea during hospitalization was associated with a prolonged hospital stay (OR 1.93 95%CI 1.19-3.13). This could be associated with Clostridioides difficile infection, a high-impact bacterium worldwide 25. Patients with CO-VID-19 may experience several of the known risk factors for Clostridioides difficile infection, such as hospital stay, ICU admission or antibiotic administration. The prevalence of Clostridioides difficile infection was not determined in this study, due to the low availability of diagnostic tests in our setting. Diarrhea can also result from direct viral damage to the enterocytes or microbiota disruptions 3. Bloody diarrhea had a very low frequency (<1%). Only 1.44% and 3.57% of the patients with COVID-19 had gastrointestinal bleeding on admission or during their hospital stay, respectively. The presence of gastrointestinal bleeding both on admission or during hospitalization was associated with worse clinical outcomes, including higher mortality (OR 3.13, 95%CI 1.1-9.1 on admission and OR 3.3, 95%CI 1.77-6.15 during hospitalization). Gastrointestinal bleeding may be secondary to severe systemic involvement due to SARS-CoV-2 infection. Upper gastrointestinal endoscopy was required for 34 patients and colonoscopy for 11, with endoscopic hemostasis only needed in three patients (one adrenaline injection for a gastric ulcer, one ligation of esophageal varices and one adrenaline injection of a peristomal colon ulcer). This suggests that most patients with COVID-19 who have gastrointestinal bleeding do not require endoscopic hemostasis; treatment would mainly involve supportive therapy, proton pump inhibitor administration, hemodynamic support, transfusions and treatment of the underlying disease. Only three patients underwent ERCP (one patient required the extraction of micro gallstones from the bile duct, one patient had a normal bile duct, and one case had no available information). An endoscopic procedure will only be necessary in select and truly urgent cases (e.g., bleeding with hemodynamic instability, cholangitis, foreign body, etc.). Also, these procedures release aerosols and can increase the healthcare staff's risk of contagion 26.
Vomiting during hospitalization was associated with respiratory failure (OR 2.41, 95%CI 1.19-4.88), shock (OR 3.54, 95%CI 1.74-14.82), ICU admission (OR 3.05, 95%CI 1.50-6.19), prolonged hospital stay (OR 2.66, 95%CI 1.32-5.36) and critical illness (OR 2.14, 95%CI 1.05-4.34). This suggests that vomiting, besides being a sign of SARS-CoV-2 infection and direct damage to the gastrointestinal tract, may also be a sign of serious systemic involvement with a higher risk of both respiratory and hemodynamic clinical deterioration.
In this study, liver profile disorders were identified both on admission as well as during hospitalization, which can help clarify if liver involvement results from direct SARS-CoV-2 action (on admission) or is multifactorial (during hospitalization), when patients are more exposed to medications, other infections, the development of sepsis, cytokine storm, shock and multiple organ dysfunction 3. Liver profile disorders were highly prevalent on admission, although not as prevalent as during the hospital stay.
The prevalence of liver profile disorders was 46%, which is within the range reported in the literature. For example, a meta-analysis showed liver profile disorders in 19% of patients with COVID-19, with a broad range from 1-53% and high heterogeneity (I 2=96%) 22.
Significant differences were found in transaminase, bilirubin and alkaline phosphatase levels according to the COVID-19 severity, with greater abnormalities in the liver profile of patients with greater disease severity (including those who died).
The most common disorders found in this study were elevated transaminases and bilirubin, with most being mild elevations. An ALT ≥ 45 U/L was found in 30.1% on admission and 40.2% during hospitalization, and an AST ≥ 45 U/L was found in 36.2% on admission and 42.6% during hospitalization. An ALT or AST > 300 U/L was only found in 0.6% and 1.1%, respectively, on hospital admission. Elevated AST on admission was associated with poor clinical outcomes like mortality and a critical course of the disease, and the strength of the association was also shown to increase as the concentration of serum AST increased. For example, having an AST > 300 U/L was associated with mortality (OR 6.8 95%CI 1.67-20.1).
Total bilirubin ≥ 1.5 mg/dL was found in 2.89% (95%CI 1.9-3.9%) on admission and 8.7% (95%CI 7.2-10.4%) during hospitalization, while only 0.6% had a total bilirubin > 6 mg/dL on admission and 1.1% during hospitalization, which was associated with respiratory failure, shock and prolonged hospital stay (OR 3.7, 4.8 and 4.5, respectively).
Alkaline phosphatase elevation was uncommon (2.5% and 5.5% of the patients with > 200 U/L on admission and during hospitalization, respectively) and generally mild (less than 0.5% of the patients had a severe elevation > 600 U/L). Elevated alkaline phosphatase was associated with higher mortality (OR 2.32 95%CI 1.09-4.95). Some studies have shown the presence of the SARS-CoV-2 S protein receptor on cholangiocytes, possibly explaining the intrahepatic cholestasis documented in these patients 27. Bile duct dilation was only found in four hospitalized patients (none on admission), although few patients underwent intra-abdominal diagnostic imaging (2.2% on admission and 3.5% during hospitalization).
This study showed that hypoalbuminemia on admission is associated with poor clinical outcomes in patients with COVID-19, including mortality (OR 8.9 95%CI 2.9-27.5 with albumin levels < 2.5 g/dL). Hypoalbuminemia may be a marker of malnutrition and is related to poor clinical outcomes. In addition, albumin has been described as a negative acute phase reactant (it decreases in the presence of inflammation) 28, which may reflect a more severe systemic involvement in patients with SARS-CoV-2 infection. No association was found between PT abnormalities and clinical outcomes.
This study's strengths include its large sample size, multicentric nature (two hospitals), the evaluation of gastrointestinal manifestations and liver profile abnormalities both on admission as well as during hospitalization, and the broad study of almost all possible gastrointestinal manifestations of COVID-19. The concentrations of transaminases, bilirubin and alkaline phosphatase were proven to be higher in patients with more severe COVID-19. Although this has also been shown in other studies 29-31, this study also determines and quantifies the risk (OR) of liver profile abnormalities for clinical outcomes, which is not often evaluated in other studies.
The limitations of this study include its retrospective nature, limited to the information recorded in the medical charts. The main limitation in determining liver profile abnormalities in this population was the large percentage of patients who did not have a full liver profile drawn, with albumin (86%), PT (80%) and alkaline phosphatase (67%) being the most frequently omitted studies. This could explain the inability to show an association between PT abnormalities and clinical outcomes.
In conclusion, the prevalence of gastrointestinal manifestations in patients with COVID-19 was 50%, with the most frequent being diarrhea, anorexia, odynophagia and abdominal pain. Diarrhea was associated with a longer hospital stay, and gastrointestinal bleeding was associated with respiratory failure and mortality. Liver profile abnormalities were found in 46% of the patients, with elevated transaminases being the most frequent. Elevated AST on admission was associated with higher mortality.











 text in
text in