Introduction
The association between COVID-19 and bradyarrhythmias has been well reported during the pandemic 1-4. Bradyarrhythmia during COVID-19 has prognostic significance 5. Cytokines/inflammatory storm, hypoxia, electrolyte imbalances and myocardial lesions are thought to contribute to arrhythmogenesis during the disease 6.
The vaccine analysis report published by AstraZeneca on April 1, 2021, reported some heart blocks, whose details are not well known 7. The literature on the presence of atrioventricular block after vaccination with COVID-19 vaccines, including BBIBP-CorV, is scant. In this report, we present a case of transient worsening of a conduction block after vaccination with COVID-19 (BBIBP-CorV) in a woman with no history of cardiovascular disease. The case report has been reported according to the SCARE 2020 criteria 8.
Case presentation
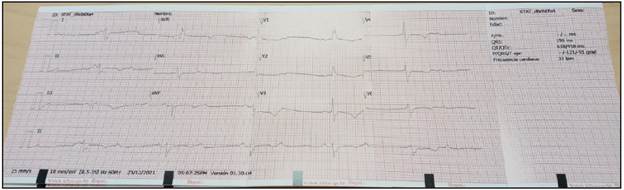
This is a case of a female in her fifth decade of life with no significant medical history, no epidemiological history of Chagas, and no family history of cardiovascular disease, who 14 days after receiving the BBIBP-CorV vaccine against COVID-19 developed functional class deterioration, multiple syncopal episodes and marked limitation in carrying out activities of daily living. She was seen by a primary care physician whose only positive finding was bradycardia ranging from 30-40 beats per minute. An electrocardiogram (Figure 1) showed complete atrio-ventricular block.
She had no signs of cardiopulmonary congestion, denied exposure to biomasses or smoking, and she was a totally functional and independent homemaker and merchant. The patient was referred to a tertiary care institution where a transthoracic echocardiogram showed a preserved ventricular ejection fraction of 60%, estimated using the Simpson method.
Immunology tests ruled out collagen disease, her levels of complement were completely normal, and endocrine diseases, including thyroid problems, were ruled out, as were Chagas and infectious processes related to bacterial or viral myocarditis.
Cardiac magnetic resonance imaging showed edema in the T2-STIR sequences and subepicardial enhancement in the medial distal lateral region compatible with acute myocarditis. An endomyocardial biopsy was proposed, but the patient refused it due to her religious beliefs.
She was referred to the cardiology service, who decided on multidisciplinary management with electrophysiology, ordering placement of a dual-chamber pacemaker. The patient had the device implanted in VVI mode and left the operating room without complications.
Discussion
Electrical or mechanical dysfunction secondary to a COVID-19 vaccine is anecdotal, with few reports in the literature. A review of both the European Society of Cardiology 2021 and American Heart Association 2018 guidelines on cardiac stimulation and cardiac resynchronization therapy found no recommendation for this type of adverse events associated with COVID-19 or after vaccination. A Phase I/II trial of the BBIBP-CorV (Sinopharm) vaccine in the Chinese province of Henan did not report any type of atrioventricular block 9. However, 29% of those who received it experienced at least one side effect within seven days of its administration. In another Phase I trial, most patients with cardiovascular disease were excluded, and no arrhythmias were reported 10. Likewise, another Phase I/II trial did not report any adverse events related to cardiac arrhythmias 11.
The concept of humoral immune response and AV block is not new. The association between maternal anti-Ro and anti-La antibodies and complete congenital heart block (CHB) is well known 12 As with the pattern of maternal antibody immune complex deposition in CHB, which causes inflammation and fibrosis in fetal conduction tissue, it is very likely that the BBIBP-CorV inactivated vaccine has a similar pathophysiological basis for causing AV block. In a prospective study of 24 anti-Ro positive pregnant women, 33% of the fetuses showed evidence of first-degree AV block, with one fetus progressing to CHB and another changing the degree of AV block from first to second degree. Another six fetuses had resolved AV block at birth or within their first 30 days of life.
Vaccine-associated AV block was reported in the literature following smallpox vaccination in 2003 13. In this particular case, a 56-year-old man was reported to have first-degree AV block and a negative cardiac biomarker on day 23 after vaccination. Likewise, intermittent incomplete right bundle branch block (RBBB) was reported in another patient after smallpox vaccination in 2009 14.
The nature of our patient's AV block after COVID-19 vaccination can be linked to the pathophysiology of heart blocks of various degrees associated with Lyme carditis. This was evident in an experiment in non-human primates injected with Borrelia burgdorferi strains showing an elevation in IgG and IgM levels in the recipients. There was a concomitant increase in complement membrane attack complex deposits 15. The reversible nature of Lyme carditis-AV block is due to the parallel reduction in the degree of inflammation over time in experimental mouse models 16. This broadens our understanding of the possible role of endomyocardial biopsy (EMB) in AV block associated with the COVID-19 vaccine. For now, EMB is not recommended for patients with COVID-19 infection and suspected myocarditis secondary to this infectious agent 17-18. However, as with its indication in early unexplainable AV blocks 19, EMB could be considered in patients with AV block after CO VID-19 vaccination. The prognostic importance of EMB in COVID-19 related AV blocks 20 needs further validation through randomized multicenter studies, as the evidence on this topic has not recently been reviewed.
Our patient started to have symptoms 14 days after being vaccinated with COVID-19. The timeline is within the first 28 days between vaccination and the onset of AV block, as well as other conduction disorders, and thus points to a direct association. Patients whose conduction system is already affected likely have a greater risk of worsening AV block after receiving the vaccine; however, this question will need to be addressed through studies and reviews.
The role of steroids and other immunosuppressants in the management of COVID-19 vaccine related AV block needs more research. Given the wide use of vaccination in the general population, including patients with underlying heart disease, it is essential to adequately evaluate these cases. Physicians should be aware of the possibility of worsening AV block after vaccination. This case is limited by not having been able to order an endomyocardial biopsy. However, there are no strong or moderate quality recommendations regarding this. The questions to be answered so far have to do with determining the treatment strategies with the best results, as studies with representative samples are lacking, and the quality of the available scientific evidence is low. For now, the patient is stable after placement of a pacemaker device from a recognized manufacturer, with optimal response. She is completely asymptomatic and has returned fully to her activities of daily life.











 text in
text in