Introduction
Ever since SARS-CoV-2 was identified at the end of 2019, it expanded rapidly throughout the whole world, giving rise to a new global pandemic due to the disease known as COVID-19 (from coronavirus disease 2019). In addition, both the clinical and epidemiological behavior of the pandemic may differ depending on the place described.
The clinical picture and prognosis of the infection varies. In mild cases, recovery occurs within the first week, and this tends to be the predominant course in 85% of infected patients 1,2. The rest of the patients may require hospital care or even progress to severe courses secondary to acute respiratory distress syndrome (ARDS) which may require admission to an intensive care unit 3,4. Likewise, severity and mortality data differ according to the geographic location. The latter is thought to range from 1.4 to 4.3%, overall 5,6. In Colombia it is around 3.0% 7. Furthermore, mortality also varies according to the clinical condition, comorbidities and age, even reaching 30 to 70% in critically ill patients 8,9.
In another vein, several factors associated with a worse prognosis or increased mortality have been described to date, including the severity of respiratory involvement, age, hypoalbuminemia, elevation of markers like C-reactive protein (CRP), lactate dehydrogenase (LDH), troponin, erythrocyte sedimentation rate, D-dimer, neutrophils, or decreased lymphocytes 2,10-12. The presence of comorbidities like arterial hypertension, diabetes and obesity are also associated with higher mortality 3,13.
Despite the efforts of several research groups, the behavior of COVID-19 in different parts of the world and in settings like intensive care must continue to be studied. In the following study, we report the data of 148 patients hospitalized in this area with SARS-CoV-2 infection, and their condition at discharge. In addition, we evaluate the potential factors associated with mortality.
Materials and methods
A retrospective cohort study was performed on patients hospitalized for COVID-19 in the ICU of Clínica Somer, which is a tertiary-care institution located in the city of Rionegro, Antioquia. The included patients needed to be at least 18 years old, with a COVID-19 infection confirmed by a real-time polymerase chain reaction (RT-PCR) test or a positive SARS-CoV-2 antigen test. In addition, the COVID-19 diagnosis had to be their main cause of admission to intensive care. A sample size was not calculated; all patients who met the study criteria throughout 2020 were consecutively included.
For data collection, Day 1 of the study was the day of ICU admission. The variables collected were age, sex, history of arterial hypertension (HTN), obesity, diabetes, dialysis, chronic obstructive pulmonary disease (COPD), chronic kidney disease (any stage), immunosuppression (secondary to diseases or medications), type of ventilatory support or assistance, and need for extracorporeal membrane oxygenation (ECMO). In addition, biochemical parameters were gathered including D-dimer, LDH, ferritin, high-sensitivity troponin, C-reactive protein (CRP), total bilirubin, leukocytes, lymphocytes, and PaO2/FiO2 (the ratio of arterial oxygen partial pressure to fractional inspired oxygen). The variables obtained were those recorded in the 24 hours before or after admission to the ICU. The collection period went from the beginning of March until December 2020. The patient's condition at clinic discharge was used for the mortality outcome. No severity scales were recorded since they were not routinely measured on ICU admission.
Regarding the statistical analysis for the description of the variables, absolute and relative frequencies were used, expressed in percentages for qualitative variables. For quantitative variables, the mean and standard deviation (SD) or median and interquartile range (the difference between the third and first quartiles) were calculated, according to their distribution. Then, the characteristics of the patients who died were compared with those who did not using a t test for the difference in means if they were normally distributed, or the Mann-Whitney U test if not. The Chi2 or Fisher's exact tests were used for categorical variables. A p < 0.05 was considered statistically significant.
To estimate the adjusted effect of the variables associated with mortality versus live discharge, a multivariate analysis was run using binary logistic regression. The entered variables were determined a priori based on a review of the known literature. Variables which were significant at a 0.3 level in the univariate analysis were included in the model. The interaction with the covariables was tested and the most significant variable was added to the model. Then, the adjusted ORs were obtained. The goodness of fit of the models was evaluated using the Hosmer-Lemeshow test.
This multivariate analysis was only applied to variables with more than 50% of the biological data available. The statistical analysis was done using R language version 3.5.2.
Regarding ethical considerations, the Declaration of Helsinki was adhered to throughout the process, and this study was also reviewed and approved by the local ethics committee in Minutes Record 34 on March 29, 2021. We also considered the privacy and confidentiality of participant data.
Results
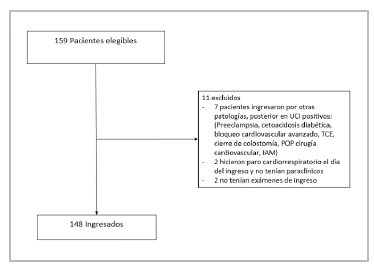
During the study period, 159 patients were admitted to the ICU with a diagnosis of COVID-19, 148 of whom met the criteria for study enrollment (Figure 1). The patients' characteristics are recorded in Tables 1 and 2. The median age was 62.5 years, and the average ICU stay was 10 days. Most were males (102, 68.9%). The most common comorbidities were HTN, obesity and diabetes. A total of 121 (81.7%) had at least one comorbidity and 137 (92.5%) of the patients met the ARDS criteria.
Table 1 Clinical characteristics of the patients admitted to the ICU for COVID-19, n=148.
| Characteristic | n (%) |
|---|---|
| Age, in years. Median (IQR)* | 62 (21) |
| Males | 102 (68.9) |
| Arterial hypertension (HTN) | 87 (58.8) |
| Obesity | 54 (36.5) |
| Diabetes | 43 (29.1) |
| Dialysis | 36 (24.3) |
| COPD** | 31 (20.9) |
| Chronic kidney disease | 19 (12.8) |
| Immunosuppression | 15 (10.1) |
| Type of ventilatory support *** Standard oxygen HFNC NIMV IMV | 11 (7.4) 5 (3.4) 3 (2.0) 129 (87.2) |
| Need for ECMO **** | 12(8.1) |
| Status at discharge Living Deceased | 45 (30,4) 103 (69.6) |
|
| |
Table 2 Biochemical parameter results on ICU admission, patients admitted for COVID-19, n=148.
| Characteristic | Data recorded, n (%) | Median (IQR) |
|---|---|---|
| High-sensitivity troponin I, ng/L | 108 (76.0%) | 55.2 (180.8) |
| D-dimer, ng/mL | 82 (57.7) | 1,739.0 (3,269.0) |
| Ferritin, ng/mL | 85 (57.4) | 1,175.5 (906.2) |
| Lactate dehydrogenase, U/L | 85 (57.4) | 442.0 (210.0) |
| Leukocytes, mm3 | 139 (97.8) | 11,430.0 (6,450.0) |
| Lymphocytees, mm3 | 139 (97.8) | 700.0 (500.0) |
| C-reactive protein (CRP), mg/mL | 19 (12.8) | 127.9 (144.0) |
| Total bilirubin, mg/dL | 136 (91.8) | 0.6 (0.4) |
| PaO2/FiO2** | 147 (99.3) | 83.0 (63.0) |
|
| ||
Regarding ventilatory support, 132 patients (89.2%) had mechanical ventilation (invasive or noninvasive). Of these, 10 (7.5%) had NIMV, seven of whom progressed to IMV due to respiratory deterioration. Twelve (8.2%) of the patients required ECMO. In the biochemical parameters, except for CRP, records were obtained for at least 84 (57%) of the patients.
A total of 103 patients died (69.6%). Of the 11 patients who required oxygen via nasal cannula or standard oxygen, only one died (9.0%); of the five patients on high flow nasal cannulas (HFNCs), none died. Finally, of the 132 patients on mechanical ventilation, 102 died (77.2%).
Regarding the patients' characteristics according to mortality outcome (Table 3), the bivariate analysis showed that the patients who died were older, with a significantly higher ferritin, LDH and CRP, and lower PaO2/FiO2 (more severe hypoxemia). A significant association was also found between the likelihood of dying and hypertension and obesity. In addition, those who required mechanical intubation had a higher likelihood of the event. While differences were found in the other recorded variables, they were not significant.
Table 3 Characteristics of patients hospitalized in the ICU for COVID-19, according to mortality outcome at discharge.
In the multivariate analysis according to the adjusted estimates (Table 4), the factors associated with higher COVID-19 mortality during the ICU stay were hypertension and elevated ferritin. In addition to the results obtained, analyses were run categorizing the variables based on what was reported in the literature. The age variable was divided into 15-year ranges beginning with age 20 (20-35 years as the reference), and PaO2/FiO2 was also grouped as < 100, 100 to 200, and more than 200 (the latter as the reference), with no significant outcomes for the two variables. Other variables listed in the previous tables were not significant in this analysis.
Table 4 Determinant factors for mortality at discharge, in patients hospitalized in the ICU for COVID-19. Multivariate analysis.
| Characteristic | Adjusted OR | 95% CI |
|---|---|---|
| Age, in years. | 1.01 | 0.95 - 1.1 |
| Males | 2.59 | 0.01 - 7.93 |
| Arterial hypertension | 3.57 | 1.29 - 4.96 |
| Obesity | 3.31 | 0.38 - 4.55 |
| Diabetes | 0.01 | 0.01 - 2.90 |
| Dialysis | 1.86 | 0.01 - 2.89 |
| COPD* | 2.66 | 0.01 - 6.33 |
| High-sensitivity troponin I, ng/L | 1.01 | 0.99 - 1.01 |
| D-dimer, ng/mL | 1.01 | 0.99 - 1.00 |
| Ferritin, ng/mL | 1.01 | 1.01 - 1.01 |
| Lactate dehydrogenase, U/L | 1.00 | 0.99 1.01 |
| Lymphocytess, mm3. | 0.99 | 0.99 - 9.99 |
| *COPD: chronic obstructive pulmonary disease. | ||
Discussion
In this study, we reviewed the characteristics of a cohort of patients who were hospitalized in the ICU for COVID-19 and their relationship with mortality measured at hospital discharge. As previously noted, the patients who died had significant differences in their biochemical and clinical parameters which can help us to continue learning about the behavior and prognosis of this disease.
The patients who died had a median age of 65 years, compared with 57 in those who survived. As consistently found in the published literature, older age is an independent factor for mortality 15. This fact has been related to aging of the immune system and its capacity to clear new infections. It is also associated with a higher prevalence of comorbidities which foster this condition's fatal outcome 16.
Most of the patients admitted to the ICU for a severe COVID-19 related condition were males (n = 102; 68.9%). While contagion is homogenous in both sexes worldwide and locally, mortality has been found to be greater in men than women. In Colombia today, 52.4% of the COVID-19 cases are in women; however, 61.1% of those who die are men 17. In 2020, the European Commission performed an analysis of the pandemic highlighting that after age 15, men have a clearly higher risk of dying, reaching a ratio of 2.5 cases in men to one in women 18. Some proposed theories lean toward a better adaptive response in women's immune systems and, on the other hand, that men may have a stronger innate response to the virus which may lead to a lethal cytokine storm 15. COVID-19 outcomes have also been found to be worse in patients with comorbidities like HTN, cardiovascular disease, and pulmonary diseases, largely associated with alcohol and cigarette use, conditions which are also more prevalent in men 19.
The admitted patients had a high prevalence of comorbidities or chronic underlying conditions. Close to 80% had at least one underlying disease. We highlight that the patients who died had a higher prevalence of HTN, obesity and diabetes (the latter without a significant effect). In the multivariate analysis, hypertensive patients had a 3.5 times greater risk of dying. An attempt has been made to clarify if hypertension per se is an independent risk factor. In some studies, the effect disappears when an age-adjusted analysis is run. However, in other studies it is associated with mortality. Its effect may be related to the probability of having more cardiovascular events in patients with severe COVID-19 15.
Regarding obesity, 43 (41.7%) of the patients who died were obese, compared with 11 (24.4%) in the group of survivors. Obesity is currently recognized as an independent risk factor associated with mortality, even in young and male patients 20. Tartof, et al. describe how, with greater obesity, the risk may be two or up to four times greater than in nonobese people 21. More severe COVID-19 in obese patients may be explained by adaptive immune response disorders, proinflammatory states, prothrombotic states, and cardio-metabolic and pulmonary function disorders. We emphasize that, even before the pandemic, visceral adipose tissue in obese patients had been reported to promote increased mortality in critically ill patients with ARDS 22.
This study also confirmed that elevated ferritin and LDH and decreased lymphocytes are related to mortality. These biomarkers are considered to reflect greater severity. A meta-analysis of 21 studies (3,377 patients and 33 laboratory variables analyzed) reported that these biomarkers, among others, were significantly higher in patients with a fatal outcome 23. Ferritin has been described as an independent factor for mortality; the level of circulating ferritin increases during viral infections and may be a viral replication marker. Regarding its pathophysiology, as noted by Chen et al. 24, many inflammatory cytokines are produced rapidly during the cytokine storm in COVID-19, including IL-6, TNF-a, IL-1ß, IL-12 and LFN-y, which stimulate the hepatocytes, Kupffer cells and macrophages to secrete ferritin. Furthermore, not only is it considered to be a marker of the inflammatory process, but it also plays a pathogenic role in the inflammatory process, activating the T cells and promoting the expression of proinflammatory mediators 25.
Lactate dehydrogenase reflects tissue damage and cell destruction, indicating an active infectious process which is causing lung damage 26. Several prognostic models indicate that it is a good biomarker for differentiating patients with a higher risk of a severe episode; it is also easy to measure, easily available and inexpensive, which makes it a good prognostic marker 26. Regarding lymphocytes, a higher level was found to be related to lower mortality. The literature consistently describes how decreased lymphocytes (lymphopenia) are associated with greater disease severity. After a systematic review, Zhao et al. noted that patients with lymphopenia had a three times higher risk of a severe episode 27. Some of the proposed mechanisms suggest cell death secondary to a cytokine storm, direct SARS-CoV-2 injury and marrow suppression, among others 27.
We found no significant differences in the other biomarkers, despite being described in the literature. This is the case with CRP and D-dimer, among others, although this could also be related to the wide variability of results and the number of records noted per patient.
Mortality was quite high in our cohort. We note that most of the patients (n=137; 92.5%) met the criteria for ARDS and 132 (89.2%) of the total patients had invasive mechanical ventilation and a very low PaO2/FiO2. This reflects the severity of their condition; in addition, during the most critical point of the pandemic in our country, there was a lack of ICU beds, healthcare staff and medications (among other circumstances) during the state of emergency, which could have had an effect, although these are conditions which should be explored in new studies.
As limitations, this study was carried out at a single center, which is also a referral center where highly complex patients were admitted, and was conducted prior to the onset of vaccination. This could lead to selection bias and may impact the external validity or generalization of the findings; however, most are in line with the known literature. On the other hand, not all biomarkers were measured in all patients, which may have limited the power to find significant differences.
Based on the results, we can conclude that COVID-19 mortality in the ICU is very high. We also documented HTN, obesity, age, ferritin, LDH, CRP and PaO2/FiO2 to be associated risk factors. However, in the multivariate model, only HTN had an effect on mortality, although this may be due to the sample size.











 texto en
texto en