Introduction
Non-Hodgkin lymphoma (NHL) is the most common hematological malignancy, with an incidence of 5-7 cases per 100,000 inhabitants worldwide, and a cumulative incidence of 544,352 in 2020 1. To date, more than 40 subtypes with different morphological, clinical and genetic characteristics have been described, making it a diagnostic challenge in which appropriate and timely treatment affects the patient's prognosis 2.
Within these subtypes, mantle cell lymphoma (MCL), or what was previously known as intermediate lymphocytic lymphoma, accounts for 3-10% of B cell NHLs, is characterized by being aggressive and usually debuts in advanced stages 3-5. An incidence of one case per 200,000 inhabitants has been reported, which increases with age, and is more frequent in Caucasians, white Hispanics and, to a lesser extent, Asians, with an average age of onset between 60 and 70 years. It is more frequent in males, with a 3:1 male to female ratio, and has a median survival of 8-10 years 4,6,7. The clinical manifestations vary; however, lymph node (75%), bone marrow (60-80%), spleen (45-60%) and ex- tranodal involvement (like the gastrointestinal tract, breast, pleura and orbit) have been reported 8-11. In addition, 33% of the patients have constitutional or B systemic symptoms like night sweats, weight loss and fever 12,13.
Once the symptoms begin, the diagnosis is based on a lymph node or bone marrow biopsy. The histopathological study evaluates the cells' phenotypic characteristics, in which small or intermediate lymphocytes, notched nuclei and blastoid cells are generally found. However, they may have fine chromatin which mimics acute leukemia, and the proliferation index and mitosis may vary 7,14. Mantle cell lymphoma may have two divisions, one known as "classic" which affects the lymph nodes and extranodal locations with a frequent expression of SOX11 and often non-mutated immunoglobulin heavy chains (IGHVs). The second or "leukemic" variant predominantly involves the blood and bone marrow, but with a generally negative SOX11 and hypermutated IGHV, and is more aggressive, with a poor prognosis 15,16. The presence of immuno-histochemichal markers like CD5, CD10, CD19, CD23, CD22 and CD25 has been reported, along with others like cyclin D1 expression. Genetic tests are considered part of the diagnostic process, with the most common being 11;14 translocation, which is found in 40-70% of the usual cytogenetic tests (karyotypes for leukemic states) and 95% of fluorescence in situ hybridization (FISH), with a lower frequency of other deletions like 11q22 and 13q14 14,17-19. In addition, diagnostic imaging has become an essential tool both for staging the involvement as well as determining the disease prognosis. Thus, positron emission tomography (PET/CT) plays a role today in the initial staging and follow up during treatment 20,21. The Mantle International Prognostic Index (MIPI), developed by the Eastern Cooperative Oncology Group (ECOG), is another instrument for the initial classification which groups three risk stages: low risk (median survival of 60 months), intermediate risk (median survival of 50 months) and high risk (median survival of 29 months). Currently, the Ki-67 (cellular proliferation) marker is included as part of this scale; however, it is operator-dependent 14,22,23.
Treatment is oriented according to the patient's classification according to age, functional status and comorbidities, which are directly related to the ability to receive intensive treatment/transplantation. Immunochemotherapy is considered for initial therapy to be consolidated later with transplantation. The treatment options include protocols with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP), rituximab, dexamethasone, cytarabine and cisplatin (R-DHAP), cyclophosphamide, vincristine, Adriamycin and dexamethasone (Hyper-CVAD) and bortezomib, rituximab, cyclophosphamide, doxorubicin and prednisone (VcR-CAP), among others. Their use depends on the MCL classification and patient characteristics 14,24. After this, the treatment should be evaluated early on, reevaluating the patient's symptoms, complete blood count, lactate dehydrogenase (LDH) and inflammatory response markers, to indirectly visualize the disease's activity. Evaluation of the extranodal sites that initially showed involvement should also be considered, for instance with gastrointestinal tract endoscopy 25,26. Finally, treatment consolidation culminates in transplantation for those with a partial or complete response. Transplantation may be autologous or allogeneic, with the choice determined by the pharmacological treatment received 27. One aspect that still causes debate is consolidation with radiation therapy, which suggests local control of the disease; however, a risk-benefit analysis is recommended in light of the side effects. These clinical interventions are currently being permeated by new treatments in which targeted therapy is now an option, with clinical studies showing its effectiveness in this type of patients.
This description of the approach to patients with MCL entails a series of both diagnostic and therapeutic challenges, which have led to a variety of alternatives with advantages and disadvantages, depending on the characteristics of the patient and the disease, which requires that the attending physicians use an appropriate approach and begin prompt, personalized treatment. These options are also tied to the availability of diagnostic resources at the different centers.
Thus, considering the relevance of this disease in terms of the patient's prognosis and care-related costs, it is important to consider standardizing the actions in order to offer the best alternatives within the Colombian context, as both diagnostic and treatment alternatives are available today, most of which are funded by the Colombian healthcare system. Consequently, the Asociación Colombiana de Hematología y Oncología (ACHO) [Colombian Association of Hematology and Oncology], which gathers a significant number of professionals involved in caring for MCL patients, has created spaces to guide real-life clinical activity. Therefore, expert multidisciplinary consensuses, like this one, have become an easily accessible and readable tool, stressing that these initiatives are not intended to replace the clinical practice guidelines, but rather present the clinical experience with this disease in an exclusively Colombian context 28.
This expert consensus on MCL considered the current legislation and regulatory agency concerns regarding the availability of and access to the diagnostic and treatment options. It is important to mention that this project is a scientific-academic rather than a regulatory initiative. Finally, this document presents some suggested medications which, despite the availability of scientific evidence, are not approved by the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) [National Institute for Drug and Food Surveillance]. These could be considered so that in the future, once they are approved, they can be used according to the indications and clinical discretion of ACHO 29.
Thus, the objective of this project was to generate an expert consensus of recommendations for diagnosing and treating MCL based on the Colombian context.
Methods
The developing group reviewed the most representative literature including primary and secondary studies, such as pivotal studies, systematic reviews and practice guidelines used by clinical experts in their usual clinical practice. The development of the consensus is described below:
PROCEDURE
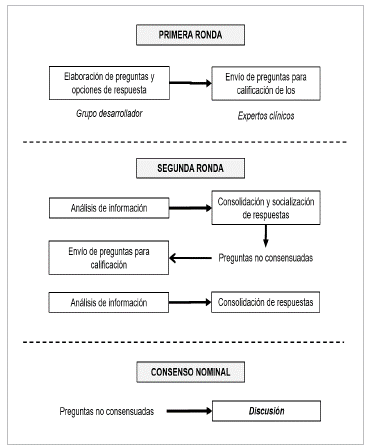
The questions were based on the importance of standardizing clinical practice, evaluating the possibility of unifying actions based on experience and the context of the Colombian healthcare system. Likewise, the group developed the questions according to each specialty's role, to obtain a position from different perspectives. A scale from 1-9 was used to score the options for each question (1 was defined as the most inappropriate or what would not be done in clinical practice and 9 was the most appropriate or what would be done as a first line action) and the interquartile ranges (IQRs) were calculated to find the dispersion of the scores. Consensus was determined when the medians were between 1 and 3 with an IQR between 1 and 3, and when medians were 7-9 with an IQR between 7 and 9. Taking this procedure into account, a matrix was designed to consolidate and analyze the results. The questions and options were constructed in Google Forms to be sent remotely to the experts (Figure 1).
First round: as described, the developing group consisting of four hematologists sent the questionnaire, which was scored by the clinical experts, and the information was then consolidated and analyzed. The options with a consensus were identified and those without consensus were sent to a second round of scoring.
Second round: a matrix was constructed showing the first-round results, which also included comments made by the experts, as part of the score. This matrix was sent to the experts in masked form to provide feedback to the entire group of experts. The form containing the questions without consensus was sent at the same time in order to reevaluate the scores according to the feedback, hoping to reach a consensus. Once the second-round scores were received, the information was consolidated and whatever definitely did not reach a consensus was sent for discussion in the nominal consensus.
Nominal consensus: the group of developers invited the experts to an open plenary to discuss the questions and options which did not achieve consensus, in order to understand the different positions and reach a consensus. In this stage, consensus was achieved when 80% of the experts agreed on an action.
Results
Twenty-five questions for diagnosing and treating MCL were drafted and discussed, with 22 medical specialists participating: 16 hematologists and hematologists-oncologists, four hematological pathologists, one radiation therapist and one nuclear medicine specialist, from Bogotá, Medellín and Cali, with a median clinical practice experience of 10.5 years, and members of ACHO.
1. WHAT HISTOPATHOLOGICALICYTOGENETIC STUDIES ARE NEEDED IN MCL ?
The following diagnostic tests are suggested:
Immunohistochemistry for CD20, CD3, CD5, CD10, CD21, CD23, BCL2, BCL6, TP53, and Ki-67, including cyclin D1.
Immunohistochemistry for SOX11.
Flow cytometry cell surface marker analysis for kappa/ lambda, CD19, CD20, CD5, CD23, and CD10.
Flow cytometry cell surface marker analysis for CD200.
FISH study for t(11;14).
It is important to consider LEF1 immunohistochemistry in the rare cases of MCL which could be confused with B-cell chronic lymphocytic leukemia (B-CLL), and in the blastoid or pleomorphic variants, as a differential diagnosis 13,27,30,31.
2. IS A LEUKEMIA KARYOTYPE ANALYSIS CONSIDERED A ROUTINE DIAGNOSTIC TEST FOR DETERMINING THE PROGNOSIS OF PATIENTS WITH MCL
A leukemia karyotype is suggested, since it is available in the Colombian setting 32.
3. ?WHAT ADDITIONAL STUDIES ARE NEEDED FOR APPROPRIATE RISK STRATIFICATION IN MCL
The following diagnostic tests are suggested: Analysis for TP53 mutation and/or 17P deletion. Analysis for IGHV mutations 4,6,14,30.
4. SHOULD THE CIRCULATING MONOCLONAL COMPONENT BE EVALUATED?
This is not routinely suggested; however, it may be considered in patients with clinical criteria and MCL variants with a monoclonal component, as well as for the initial differential diagnosis.
5. REGARDING FULL-BODY PET/CT AT DIAGNOSIS:
The following is suggested:
Initial staging with PET/CT for ALL cases, especially at diagnosis, if radiation therapy or shortened systemic therapy is planned for treating stages I and II (early), as this may affect the radiation therapy prescription and length of treatment.
Ordering a PET/CT should not prevent beginning medical or surgical treatment, considering the time required to perform the test and receive the results, as well as its availability 13,20,21,31,33-36.
6. REGARDING UPPER GL ENDOSCOPY AND TOTAL COLONOSCOPY WITH A BIOPSY AND SEDATION:
This is always suggested in ALL cases at diagnosis, and is especially important in stages I and II, when there are gastrointestinal symptoms and/or when there is gastrointestinal bleeding.
This suggestion was agreed upon by the participating experts based on their experience, coinciding in the perception of a greater prevalence of possible gastrointestinal involvement in this type of lymphoma in our country. This is why it is recommended for ALL cases, as long as there is access to endoscopic studies, keeping in mind that PET/CT is not available in some geographical areas. However, according to the NCCN guidelines, this varies according to the epidemiological and access situation in some regions of Colombia 12,13,27,37.
7. REGARDING BONE MARROW ASPIRATION/BIOPSY STUDIES
These are recommended for all cases at diagnosis, especially for stages I and II with hematological involvement found on the complete blood count 13,27,38.
8. REGARDING LUMBAR PUNCTURE FOR CEREBROSPINAL FLUID (CSF) STUDIES (LIKE CYTOCHEMISTRY, CYTOLOGY, AND FLOW CYTOMETRY IN A TRANSFIX TUBE)
The recommendation is:
For patients with a risk of central nervous system involvement (blast variant with central nervous system symptoms, proximity to the central nervous system, involvement of the ocular adnexa, kidney involvement and elevated LDH)
It may be considered in other cases, according to clinical judgement and the availability of diagnostic tests 12,14,27.
9. REGARDING IMAGING ASSESSMENT OF THE TREATMENT RESPONSE
It is suggested that:
Full-body PET/CT be considered for the final evaluation of treatment in all cases.
Chest, abdominal and pelvic tomography with contrast should be considered for ALL cases in which they are not contraindicated, if PET/CT is not performed.
Routine full-body PET/CT is not suggested for intermediate evaluation during treatment. However, it may be considered in those who had a positive initial PET/CT, and to evaluate extranodal involvement.
It is important to mention that ordering a PET/CT should not be an impediment to continuing medical or surgical treatment, considering the time required for it to be performed and have results delivered, as well as its availability 13,20,21,31,33-36.
10. REGARDING REPEATING THE BIOPSY AND ANALYSIS OF THE TP53 MUTATIONI17P DELETION:
It is suggested:
For symptom progression or the onset of indications for treatment of the indolent type. For all cases of nonresponse or relapse 27.
11. WHAT FIRST LINE TREATMENT OPTIONS DO YOU CONSIDER FOR PATIENTS WITH EARLY MCL DISEASE (STAGE IUI NON-BULKY) ?
The following is suggested:
Shortened chemo-immunotherapy, consolidated with radiation therapy.
In localized disease, in those in whom chemotherapy cannot be used or for patients who prefer not to use chemotherapy (unsuitable for systemic treatment), exclusive radiation therapy may be considered as an option 27,39.
12. IF USING CHEMO-IMMUNOTHERAPY, WHICH FIRST LINE TREATMENT PROTOCOL WOULD YOU CONSIDER FOR PATIENTS WITH EARLY MCL DISEASE (STAGE IUI NON-BULKY)?
The following are suggested:
R-CHOP.
R - Bendamustine is considered to be a treatment option; however, it does not have INVIMA registration at the moment, despite evidence of its use. VcR-CAP is considered as a treatment option in Stage II (according to clinical judgment). It is not suggested in Stage I and shortened regimens.
The following is not suggested: Rituximab + lenalidomide 27.
13. WHAT FIRST LINE TREATMENT OPTIONS WOULD YOU CONSIDER FOR PATIENTS WITH ADVANCED AGGRESSIVE MCL (II BULKY - STAGES III AND IV) WHO ARE CANDIDATES FOR TRANSPLANTATION - INTENSIVE TREATMENT?
The following are suggested: Alternating R-DHAP / R-CHOP.
R - DHAP. Hyper-CVAD.
NORDIC regimen: Maxi-CHOP.
The following are not suggested:
R-CHOP. VcR - CAP.
R - Bendamustine 27.
14. WHAT FIRST LINE TREATMENT OPTIONS WOULD YOU CONSIDER FOR PATIENTS WITH A DIAGNOSIS OF ASYMPTOMATIC INDOLENT MCL?
Suggested: Observation.
Not suggested:
15. WHAT FIRST LINE TREATMENT OPTIONS WOULD YOU CONSIDER FOR PATIENTS DIAGNOSED WITH ASYMPTOMATIC INDOLENT MCL WITH AN INDICATION FOR TREATMENT WHO ARE CANDIDATES FOR TRANSPLANTATION - INTENSIVE TREATMENT ?
The following are suggested as treatment options: Modified hyper-CVAD. Alternating R-DHAP / R-CHOP.
R - DHAP.
Hyper-CVAD.
NORDIC regimen: Maxi-CHOP.
In the event that one of the previous options is not indicated or is contraindicated, the following is suggested: Bendamustine + rituximab.
It is important to keep the patient's comorbidities and tp53 mutation in mind.
The following is not suggested: Rituximab - lenalidomide.
VcR-CAP.
It is important to consider that indolent is defined as: not rapidly progressive, without blastoid morphology, patients with negative SOX11 and p53 biomarkers, mutated IGHV, a clinical presentation similar to non-nodal CLL, having splenomegaly, a low tumor burden and Ki-67 <30% (13, 27, 40, 42).
16. WHAT FIRST LINE TREATMENT OPTIONS WOULD YOU CONSIDER FOR PATIENTS DIAGNOSED WITH ASYMPTOMATIC INDOLENT MCL WHO ARE NOT CANDIDATES FOR TRANSPLANTATION OR INTENSIVE TREATMENT?
Suggested: R-CHOP. R- Bendamustine.
VcR-CAP
Rituximab + bendamustine + citarabine (R-BAC 500).
Not suggested: Rituximab - lenalidomide. Observation (13, 27, 40, 41).
17. IN WHICH PATIENTS IS RADIATION THERAPY INDICATED ?
Suggested in:
Bulky disease, in patients who have finished chemo-immunotherapy and have it indicated as consolidation. Localized disease.
Patients with a partial response, who are not candidates for transplantation.
° It should be kept in mind that in cases where the patients do not respond to their first line and are not candidates for transplantation, the biopsy should be repeated and a second line of rescue treatment should be started 27,39.
18. SHOULD CONSOLIDATION WITH AUTOLOGOUS HEMATOPOIETIC STEM CELL TRANSPLANTATION BE DONE AT THE FIRST REFERRAL IN ELIGIBLE PATIENTS?
Consolidation with autologous hematopoietic stem cell transplantation is suggested at the first referral of fit patients 13,27.
19. SHOULD MAINTENANCE STRATEGIES BE USED IN MCL PATIENTS OVER THE AGE OF 18 WITH COMPLETE RESPONSE TO THE INITIAL TREATMENT?
Maintenance strategies are suggested in MCL patients over the age of 18 with complete response to the initial treatment.
It is important to mention that maintenance applies both to cases that undergo high-dose therapy and autologous hematopoietic stem cell transplantation (AHSCT) after this complete treatment response as well as those that do not undergo AHSCT, especially if the R-CHOP regimen was their initial treatment 27.
20. WHAT TREATMENT IS RECOMMENDED FOR MAINTENANCE IN PATIENTS WITH MCL?
Suggested:
Rituximab in patients who had a complete response. Not suggested:
21. WHAT MCL TREATMENT OPTIONS WOULD YOU CONSIDER IN PATIENTS WITH CENTRAL NERVOUS SYSTEM INVOLVEMENT?
Suggested:
Intrathecal chemotherapy. High-dose methotrexate.
Palliative radiation therapy in patients with no other treatment option 27,39,43.
22. WHAT RESCUE TREATMENT WOULD YOU CONSIDER IN PATIENTS FIT FOR INTENSIVE TREATMENT WITH A CONFIRMED MCL DIAGNOSIS ON THEIR FIRST RELAPSE?
Suggested: Ibrutinib.
R-DHAP, if it was not used as first line treatment. Rituximab - ifosfamide, cytarabine, etoposide (R-ICE). RBAC-500 in patients with late relapse and in good clinical condition. R-Bendamustine.
Rituximab - gemcitabine, oxaliplatin (R-GEMOX).
° It is important to mention that this should not be considered for first line intensive consolidation treatment.
In patients who are to undergo allogeneic transplant or a second autologous transplant, more intensive regimens should be considered for rescue, according to the patient' s clinical condition.
Not suggested:
23. WHAT RESCUE TREATMENT OPTIONS WOULD YOU CONSIDER FOR PATIENTS WITH A CONFIRMED MCL DIAGNOSIS WHO ARE NOT FIT FOR INTENSIVE TREATMENT, ON THEIR FIRST RELAPSE?
Suggested:
Ibrutinib.
R-bendamustine.
Not suggested:
R-DHAP.
R-ICE.
R-GEMOX.
R-CHOP (27, 45, 47).
24. WHAT IS THE ROLE OF ALLOGENEIC HEMATOPOIETIC STEM CELL TRANSPLANTATION IN PATIENTS IN SECOND REMISSION?
Allogeneic hematopoietic stem cell transplantation can be considered for eligible patients in second remission 27,48-50.
Glossary
IGHV: immunoglobulin heavy variable
PET/CT: positron emission tomography /computed tomography
R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone
R-Bendamustine: rituximab - bendamustine
VcR-CAP: rituximab, cyclophosphamide, doxorubicin, bortezomib, and prednisone
R-DHAP: rituximab, dexamethasone, cytarabine and cisplatin
Hyper-CVAD: cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate and cytarabine
MaxiCHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone
R-BAC 500: rituximab, bendamustine and cytarabine,
R-ICE: rituximab, ifosfamide, carboplatin and eto-poside
R-GEMOX: rituximab, gemcitabine and oxaliplatin











 text in
text in