Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.41 no.1 Bogotá Jan./Apr. 2012
PRODUCTION AND PURIFICATION OF IgY ANTIBODIES AS A NOVEL TOOL TO PURIFY THE NR1 SUBUNIT OF NMDA RECEPTOR
PRODUCCIÓN Y PURIFICACIÓN DE ANTICUERPOS IgY COMO UNA HERRAMIENTA NOVEDOSA PARA PURIFICAR LA SUBUNIDAD NR1 DEL RECEPTOR NMDA
PRODUÇÂO E PURIFICAÇÂO DE ANTICORPOS IgY COMO UMA FERRAMENTA INOVADORA PARA PURIFICAR O RECEPTOR NMDA SUBUNIDADE NR1
Edgar A. Reyes-Montaño1,3, Leonardo R. Lareo2, Gerardo Pérez1
1 Universidad Nacional de Colombia, sede Bogotá, Facultad de Ciencias, Departamento de Química, Grupo de Investigación en Proteínas (GRIP). Av Cra 30 45-03- Bogotá D.C., Código Postal 111321 - Colombia.
2 Pontificia Universidad Javeriana, Nutrition and Biochemistry Department, Computational and Structural Biochemistry and Bioinformatics Research Group.
In memoriam
Recibido: 10/11/11 - Aceptado: 30/04/12
ABSTRACT
Producing polyclonal antibodies (IgY) in chickens has advantages over those obtained in other animal models, since they have been used as a tool for studying different proteins (NMDA glutamate receptor in our case, specifically the NR1 subunit). We produced specific antibodies against expression products by the alternative splicing of the gene encoding NMDA receptor NR1 subunit in adult rat brain. Three peptides corresponding to the splicing sites (N1, C1 and C2' cassettes) were designed, synthesised and used individually as antigens in hens. Specific immunoglobulins were purified from yolks. The antibodies were then used for purifying the NMDA receptor NR1 subunit using affinity chromatography coupling the three antibodies to the support.
Key words: IgY, antibody, glutamate, NMDA, NR1 subunit, affinity chromatography, purification.
RESUMEN
La producción de anticuerpos policlonales en gallinas (IgY) tiene ventajas sobre anticuerpos obtenidos en otros modelos animales y se han empleado como una nueva herramienta para estudiar diferentes proteínas (el receptor de glutamate tipo NMDA en nuestro caso, específicamente la subunidad NR1). Produjimos anticuerpos específicos contra productos de expresión por splicing alternativo del gen que codifica la subunidad NR1 del receptor tipo NMDA en el cerebro de rata adulta. Se diseñaron 3 péptidos correspondientes a los sitios de splicing del gen (conocidos como casetes N1, C1 y C2'), se sintetizaron y se usaron individualmente como antígenos en gallinas. Inmunoglobulinas específicas se purificaron de las yemas. Los anticuerpos se usaron para purificar la subunidad NR1 del receptor tipo NMDA usando cromatografía de afinidad, a través del acople de los tres anticuerpos al soporte.
Palabras clave: IgY, anticuerpo, glutamato, NMDA, subunidad NR1, cromatografía de afinidad, purificación.
RESUMO
A produção de anticorpos policlonais (IgY) em galinhas tem vantagens sobre os obtidos em outros modelos animais e eles têm sido usados como uma ferramenta para o estudo de proteínas diferentes (NMDA receptor de glutamato no nosso caso, especificamente a subunidade NR1). Nós produzimos anticorpos específicos contra produtos de expressão pela splicing alternativo do gene que codifica receptor NMDA subunidade NR1 no cérebro de ratos adultos. Três peptídeos correspondentes aos locais de splicing (N1, C1 e C2' cassetes) foram concebidos, sintetizados e utilizados individualmente como antígenos em galinhas. Imunoglobulinas específicas foram purificadas a partir de gemas. Os anticorpos foram então usados para purificar o receptor NMDA subunidade NR1 utilizando cromatografia de afinidade, por meio da junção dos três anticorpos ao suporte.
Palavras-chave: IgY, anticorpo, glutamato, NMDA, subunidade NR1, cromatografia de afinidade, purificação.
INTRODUCTION
Chickens IgG (IgY) have some physicochemical characteristics differentiating them from mammals IgG (1). For example, they do not interact with rheumatoid factors, do not activate the human complement system, do not bind to Staphylococcus sp. protein A, protein G nor to mammalian cells Fc receptors in such a way that the probability of false positives being given in immunological assays becomes greatly reduced (2, 3, 4).
In nature, IgYs are found in two forms: one is complete and the other truncated. Both can coexist in a single individual as happens in some turtles and in ducks (5). The complete form has a 180 kDa molecular mass and (like all low weight immunoglobulins) two heavy (H) and two light (L) chains. The H chains have both variable (V) and constant (C) regions with four immunoglobulin domains, thus explaining their large size (6). The truncated form has a 120 kDa molecular weight as it has lost its CH3 and CH4 domains from the H chains, making into an F(ab')2-like fragment molecule.
Ionotropic Glutamate receptors are directly related to long-term potentiation (LTP) processes such as memory and learning. This type of receptor has been subdivided into three groups dueto the use of different external agonists; these groups are known as N-Methyl-DAspartate (NMDA), AMPA and Kainate, they seem to have great similarity structurally and functionally, even though the processes in which they intervene could be different or complementary.
The NMDA receptor consists of several subunits known as NR1, NR2 and NR3, the first two being the most studied due to their greater presence in functional receptors. Previous studies have shown that the receptor functions as a channel as long as it has at least one NR1 subunit in its conformation (7).
The NR1 subunit has eight isoforms due to the presence of alternative splicing in three of the exons of the gene producing this protein. These segments are called N1, C1 and C2 even though the latter might be present or not, leading to the appearance of an additional segment called C2' which is only found in case C2 is not present (7).
MATERIALS AND METHODS
The segments corresponding to each NR1 subunit splicing site were identified (N1, C1 and C2' cassettes). Peptides corresponding to each of them were then designed. These peptides were synthesised at Fundación Instituto de Inmunología de Colombia synthesis laboratory (FIDIC) and used as antigens for producing IgYs. Once the peptides had been obtained, Hi- Line Brown hens were immunised.
Peptide synthesis
Each peptide was synthesised by the solid phase method, following Bocstrategy in polypropylene bags (Biotech. Instruments, USA), using 4-methylbenzylhydrilamin resin (MBHA) (100-200 mesh, 1-1,2 mmol/g, Fluka, Switzerland) (8). Boc-Lys(Fmoc)-OH (BACHEM, Switzerland) was used in the synthesis in the peptide's amino terminal position to avoid modifying this residue's side chain group. Temporary protection (Boc), including that of the NH2-terminal, was eliminated by treatment with 37.5 % trifluoroacetic acid (TFA) in dichloromethane (DCM). 0.2 M acetic acid in DCM was used for acetylating the peptide's amino terminal following activation coupling with 1,3-diisopropylcarbodiimide (DIC) methodology. Low-High HF procedure was used for final deprotection; pure HF (fluorhydric acid) was used for analysis. The bag was then washed with diethyl ether and vacuum-dried. The peptide was extracted from crude final product with 30 % acetic acid (AcOH). The final extract was diluted with water and lyophilised. Synthesis products were determined by high resolution liquid chromatography (HPLC) using a 5-60 % acetonitrile gradient (0.1 % TFA) in water for 30 min. the peptide was detected by its absorbance in ultraviolet and it was characterised by MALDI-TOF mass spectrometry (Voyager), using a lineal operation mode (2,000V acceleration voltage and 100 shots per spectrum).
Using peptides as antigens
Twelve 16-week old hens (3 per peptide and 3 controls) were used which had been conditioned for two weeks in individual cages with food available ad libitum. Once this time had elapsed the animals were kept in the same conditions until they began to lay eggs and their production became regularised at 1 egg per day (at around week 20). The peptides began to be inoculated at this time. The chickens were immunised (9, 10), the antigen having been previously emulsified; this involved preparing solutions of each peptide (1.8 mg dissolved in 2 ml 50 mM phosphate buffer, pH 7.5) to which 2.5 ml Freund's complete adjuvant (Sigma) was slowly added, homogenising it later on. This solution (around 1.0 ml) was intramuscularly injected into several areas of the pectoral region. Two boosters were applied using the same amount of peptide at 10-day intervals using Freund's incomplete adjuvant.
The eggs were collected daily and kept at 4 °C until being used in extraction. The selection was made according to the peptide used as antigen and the days post-inoculation.
Extracting and purifying IgY
Lipids were removed from the yolks following the previously described method (11), a precipitate being obtained at the end of the process mainly consisting of lipids and a supernatant in which most proteins were found, including the IgY. 60 % s (NH4)2SO4 was used with constant shaking at 4 °C for 3 hours for precipitating the proteins from the supernatant. Once this time had elapsed, the solution was centrifuged at 18,000 rpm for 30 minutes at 4 °C; the pellet was suspended in distilled water (about a tenth part of the initial volume) and kept at -20 °C.
The activity of the antibodies present in the eggs from the first day of inoculation was determined by ELISA assay (12) using the corresponding peptides as antigens (5 mg in carbonate buffer, pH 9,6); the protein extracts so obtained (IgY) were not diluted. The samples presenting the greatest activity were used for purifying total IgY; this was done by thiophilic chromatography using adsorbent T-gel as support (Pierce ™) (12, 13). The columns used were packed and activated according to the manufacturer's recommended procedure. The columns were then equilibrated with four volumes of binding buffer (0.5 M Na2SO4, 50 mM Na3PO4, pH 8.0, 0.05% NaN3). The sample was dissolved in 0.5 M Na2SO4 and allowed to totally soak into the gel. The column was washed with 13 volumes of binding buffer, collecting around 3 ml fractions and monitoring the presence of non-retained material by absorbance at 280 nm. About 12 volumes of elution buffer (50 mM Na3PO4, pH 8.0, 0.05% NaN3) were used for eluting the IgYs (retained material), determining protein presence by reading absorbance at 280 nm. The support was regenerated according to the manufacturer's recommendations. The fractions so obtained were analysed by electrophoresis (PAGE-SDS) according to (14).
Affi-Gel-10 and Affi-Gel-15 (Bio- Rad) were used for purifying specific IgYs; they were separately coupled (15) to each of the corresponding peptides according to the manufacturer's recommendations (aqueous coupling). 2 ml Affi-Gel were washed with 3 volumes of cold distilled–deionised water (4 °C). The support was then equilibrated with sodium phosphate buffer (0.1 M NaH- 2PO4, 0.1 M Na2HPO4, pH 7.5) which was used as ligand solvent. 0.5 ml of the solution containing ligand (1 mg/ml) was added and kept overnight with constant shaking. Once this time had elapsed, the column was packed and washed with the same buffer. The procedure above was repeated until absorbance was found on the base line again, but 0.1 M Gly in phosphate buffer was used instead of peptide as blocking agent for binding sites which had not been occupied by the peptide.
The columns were washed with phosphate buffer and equilibrated with buffer PBS (0.1 M NaH2PO4, 0.1 M Na2HPO4, 150 mM NaCl, pH 7.5); the IgY samples were then applied (close to 2 mg of protein) dissolved in the same equilibrium buffer. The sample was eluted with PBS and the material retained was eluted with 0.5 M AcOH when all the non-retained material had been obtained, 1 ml fractions being collected.
The support was washed with cold deionised water and conserved in 0.1 M sodium phosphate buffer, pH 7.5 at 4 °C. The retained fractions (IgY–antipeptide) were concentrated by ultrafiltration (Amicon YM 10), evaluated by ELISA assay (using peptides as antigens) and by PAGE-SDS following transfer to nitrocellulose membrane; protein concentration was determined by the bicinchoninic acid method (BCA) (16). The fractions were transferred to nitrocellulose membrane (45 µm, BioRad) according to (17), using 48 mM Tris, 39 mM Gly, 20 % methanol, pH 9.3 as transfer buffer, 80 minutes transfer time at 100 V and 350 mA. Once this time had elapsed, the nitrocellulose membranes were blocked overnight with 5 % PBS-skimmed milk at 4 °C and then washed thrice with PBS- 0.1 % Tween 20, followed by a 2-hour incubation at 37 °C with the corresponding secondary antibody (peroxidase-coupled anti-hen IgG, Sigma) diluted 1:2,000 in 5 % PBSmilk, for 2 hours at 37 °C. They were washed again with TBS-Tween 20 and 30 ml fresh revealing solution was added (15 mg tetrahydrochloride 3,3'-diaminobenzidin (DAB) in 30 ml PBS buffer, pH 7.2, plus 30 µL H2O2).
Once the presence of IgYs had been demonstrated, their specificity for recognising NR1 subunit was determined; PAGE-SDS of crude rat brain extract was thus carried out and transfer to nitrocellulose membrane was done according to previously mentioned conditions. Following blocking with 5 % PBS-skimmed milk, the corresponding washes with PBS-Tween 20 were done and each membrane was incubated overnight with the primary antibodies (previously purified anti-N1, anti-C1 and anti-C2' were used separately) at 4 °C. Each membrane was then washed twice with PBS-Tween 20 followed by 2-hour incubation at 37 °C with the corresponding secondary antibody (peroxidase-coupled anti-hen IgG, Sigma) diluted 1:2,000 in 5 % PBSmilk for 2 hours at 37 °C. They were washed again with TBS-Tween and 30 ml fresh revealing solution were added (15 mg DAB in 30 ml PBS buffer, pH 7.2, plus 30 µL H2O2) (18).
Purifying NMDA receptor NR1 subunit
The 3 anti-peptide antibodies were coupled to the Affi-Gel support in the same conditions previously described for coupling the peptides (15). The amount of antibodies was around 600 µg (200 µg of each purified antibody) coupled to 2 ml resin and coupling proceeded for 12 hours at 4 °C with circular movements. Once this time had elapsed, the support was washed with PBS pH 7.5, glycine solution was added (0.1 M in PBS) and it was incubated for 12 hours at 4 °C with circular movements. The support was then washed and equilibrated with 0.1 % Triton X-100 in PBS buffer, pH 7.5.
The sample (adult rat brain extract prepared according to 18), previously dialysed against the equilibrium buffer, was put on the column (1 ml sample (3.5 mg/ml)) and incubated for at least 2 hours at 4 °C (or overnight in some cases). Once this time had elapsed, the nonretained material was eluted with equilibrium buffer (PBS pH 7.5, 0,1% Triton X-100) until absorbance at 280 nm again reached the base line. The retained material was then eluted with 0.1 M Gly pH 2.4, 0.1 % Triton X-100, the presence of protein being monitored by absorbance at 280 nm. The fractions so collected (800 µL each) were immediately neutralised by adding 200 µL 0.5 M Tris pH 8.0. Once the whole fraction retained from the column had been eluted, it was washed and equilibrated with PBS buffer pH 7.5 for regenerating the support.
The fractions were collected and concentrated by ultrafiltration using an Amicon YM-10 membrane. Once these fractions had been concentrated, SDS-PAGE assays were carried out on them and they were transferred to nitrocellulose paper for determining the presence of NR1 subunit. Transfer was done in the aforementioned conditions; purified IgY was used as primary antibody (1:500 in 5 % PBS-milk) and commercial peroxidasecoupled anti-hen IgG (Sigma) (1:2,000 in 5 % PBS-milk) as secondary antibody.
Incubation, washing and revealing times were set according to the aforementioned. A commercial primary antibody was also used as positive control (antiNR1 produced in goat, Santa Cruz 1:1,000 in 5 % PBS-milk) and, in this case, the secondary antibody used was peroxidasecoupled rabbit antigoat (Sigma) (1:2,000 in 5 % PBS-milk).
RESULTS AND DISCUSSION
Designing and synthesising peptides used as antigens
Chickens represent a very suitable source of antibodies (IgY) as well as offering an easier, faster and convenient way of obtaining antibodies without sacrificing animals and ensuring to maintain an almost regular production after boosters have been applied (19). The greater phylogenetic distance was also taken into account when selecting the animal model, since birds should be a good model for producing specific antibodies if the NMDA receptor were found to be conserved amongst mammals. The search regarding the presence of NR1 subunit in Gallus gallus revealed only sequences corresponding to computational predictions of subunit NR2B (XP416204), NR3B (XP426726), NR2D (XP427751)- like proteins; however, no reports were found for the NR1 subunit sequence in this organism.
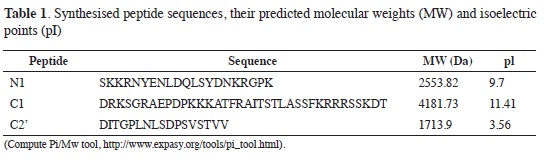
Each subunit isoform sequence has been reported in GenBank (http://www.ncbi.nlm.nih.gov/Entrez) as a conceptual translation of messenger RNA found in different areas of the brain. Multiple alignment (ClustalW) was used for determining the sequence corresponding to each splicing-site product, these being the basis for designing the peptides used as antigens for producing polyclonal antibodies (Table 1).
These peptides were selected since predicting antigenicity (20) could produce a better immune response in the animals which were inoculated. According to secondary and tertiary structure prediction it was possible that their folding led to their great ability to produce an immune response since they probably had an α-helix configuration leading to several of their amino acid side chains interacting with the medium, thereby producing a good immune response.
Peptides N1, C1 and C2' were synthesised at FIDIC following the above analysis. MALDI-TOF mass spectrometry N1 peptide characterisation revealed the highest HPLC peaks (close to 95 %) corresponding to 2,554.9, 2,572.9 and 2,663.7 Da species, having 1.1 Da, 19.1 Da and 109.9 Da difference, respectively, respecting the peptide's calculated molecular weight (2,553.8 Da). Such differences could have been due to the presence of a proton (1.1 Da), 1 water molecule plus a proton (19.1 Da) and 6 water molecules (109.9 Da).
The mass spectrum obtained for peptide C1 showed that the greatest intensity compound presented species having 4,208.9 Da, 4,214.8 Da and 4,219.1 Da molecular weights, having 27.2 Da, 33.1 Da and 37.4 Da differences, respectively, regarding predicted molecular weight (4,181,7 Da). Such differences could have been due to contaminant metallic ions, water molecules and protons being present in the peptide when determining its molecular weight. The largest compound for peptide C2' had a 1,731.2 Da molecular weight, presenting a difference of 17.3 Da regarding predicted molecular weight (1,713.9 Da) which could have been due to the presence of water molecules.
Designing peptides from N1, C1 and C2' segments corresponded to sequences which had been previously reported (21), even though a difference in an amino acid was presented in the C2 peptide proposed by ourselves. Their great tendency to fold themselves into α-helices became apparent when predicting each peptide's tertiary structure, a situation which was not strange as this is one of the most stable conformations which a short amino acid sequence can adopt, such as that existent in each segment. However, due to their short extension, it could not be ignored that the structure might adopt a different form in the aqueous medium in which amino acids are dissolved. If it were accepted that the tridimensional structure were so, then this would be a good factor for amino acid side chains to interact with the medium in which they are found and produce a good immune response.
Peptide antigenicity was also predicted using Abie Pro 3.0: Peptide Antibody Design 8 software (http://www.changbioscience.com/abie/abie.html); it was found that peptide N1 was probably antigenic since it presented 9 highly antigenic internal segments (22), or 2 according to Kyte and Doolittle's scale (23). 9 and 4 segments were present for C1 (according to the same scales) but only one antigenic site was present in C2', even though its extension was greater than that of the segments predicted for the other peptides. However (according to all the results) the peptide having the greatest probability of being antigenic was C2' since it presented the greatest percentage of amino acids which could become antigenic due to their location and hydrophobicity.
Extracting IgY The chickens were immunised after the 10th egg-laying day, such time being when they all maintained regular production; thereby, inoculation did not affect production leading to the deduction that animal-handling was suitable and did not produce a high degree of stress in them. No alteration was observed in the animals' egg-laying when the two boosters were applied.
The procedure used led to extracts being obtained having 5 to 10 mg/ml protein concentration and an electrophoretic profile mainly revealing 60 to 70 kDa and 20 to 30 kDa molecular weight proteins (data not shown). These results were obtained for all the extracts, indicating the procedure's convenience for obtaining IgYs from egg-yolks.
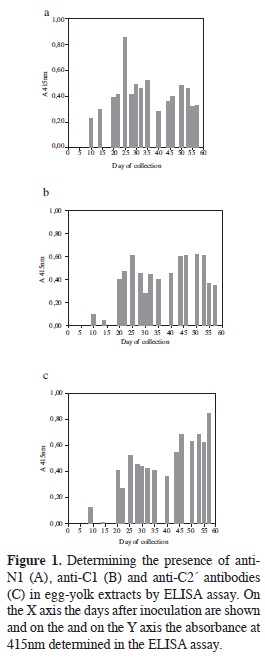
ELISA was used for determining the presence of specific IgY against the three peptides using samples from eggs collected from the day after inoculation, counting this as being day 1. The results obtained from the samples from the first ten days showed that IgY concentration tended to increase towards the 8th day of beginning to produce specific antibodies against the inoculated peptides (data not shown).
Figure 1 shows the response obtained for the whole egg-collecting period. A maximum response was observed for peptide N1 towards day 25 (Figure 1A), followed by a decrease but recovering following day 40 (20 days after the 2nd booster). Peptide C1 (Figure 1B) presented behaviour in which there was a transitory increase towards day 25 and then the response remained constant (at lower levels) until day 55. The highest responses were obtained with peptide C2' (Figure 1C) 20 days after the second booster, such response being maintained until the end; this result ratified predicted antigenicity according to which C2' was shown to be the most antigenic of the selected peptides.
When the peptides were used as antigens, the presence of antibodies was directly identified in the egg-yolk. As the yolk consists of around 50 % of material which is insoluble in water, the first step in extracting IgY involved separating soluble proteins in water from the rest of the material found in the yolk. This process has been described using different buffers (9, 11) or an aqueous dilution of acetic acid (9, 24); however, in our case we opted for extraction with water following results obtained by (12), HCl being added until 5.0 pH was obtained, since IgY were thereby recovered eliminating the greatest possible amount of lipids and hydrophobic molecules, obtaining a clear solution having a high concentration of proteins presenting bands characteristic of IgY H and L chains (12, 24). Adding 60 % s (NH4)2SO4 to supernatant caused precipitation of thiophilic chromatography- purified IgY (13, 25) facilitating obtaining specific IgY.
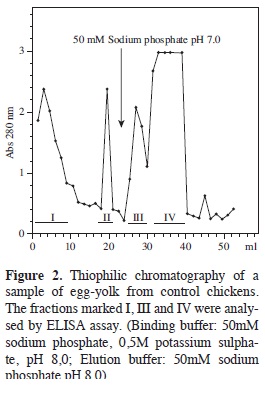
Purifying total IgY IgY was separated from control hens yolks (not having been inoculated) in an initial assay, observing (Figure 2) that the amount of IgY in the extract was high since the fractions retained by the T-Gel Similar chromatographic profiles were observed for the extracts from immunised hens. It was observed in the case of the extract called N1 (non-purified IgY anti- N1pool) that the retained fractions absorbance value was around 1.3, indicating high protein content. A similar pattern was obtained with extracts C1 and C2', retained fraction's absorbance reaching values around 0.8. In these three cases, the greatest variation was presented in the area of elution of the material which was not retained by the support, since some cases presented just a single peak having very high value and others presented several peaks having intermediate absorbance values. The presence of proteins having similar molecular weights to the IgY H and L chains were revealed in the retained fractions by PAGE- SDS.
Purifying specific IgY
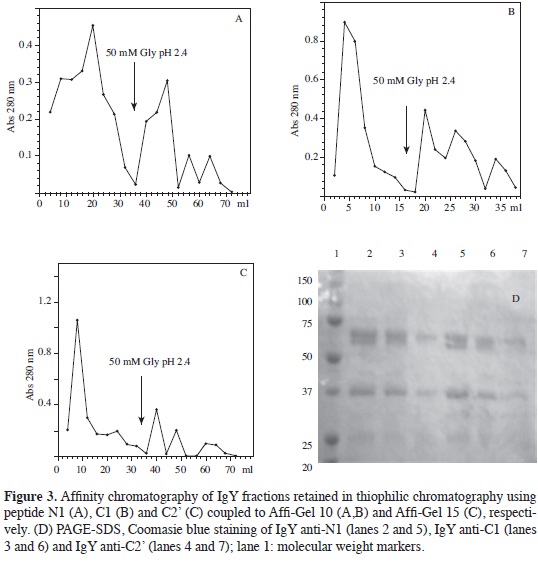
IgYs directed against peptides were purified by affinity chromatography using N1-AffiGel 10, C1-AffiGel 10 or C2'- AffiGel 15 as supports. The amount of recovered protein (according to each retained fractions absorbance, see Figure 3) was very similar in all three cases since maximum value was close to 0.3 absorbance units for all of them. It is worth noting that in all assays carried out the retained fraction presented 3 to 4 poorlyresolved peaks presenting activity by ELISA assays, meaning that they were put into a single pool.
Electrophoresis of the retained and non-retained fractions for the three thiophilic chromatographies (Figures 3A, 3B, 3C) revealed that immunoglobulin concentration in the non-retained fraction was much greater than that present in the fraction retained, which was to be expected given that they corresponded to non-specific IgY.
Pools from different chromatography (done with the same ligated peptide) were put together to better show proteins present in the retained fraction; these were concentrated 50-fold by ultrafiltration and analysed by PAGE-SDS. The bands corresponding to the H and L chains of each specific IgYs produced may be seen in Figure 3D.
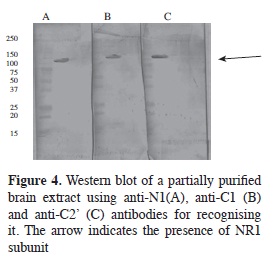
Alternative splicing products were recognised by specific antibodies for each of them in Western blot assays on adult rat brain extract (Figure 4) where the binding of the three produced antibodies (IgYs) to proteins having close to 130 kDa molecular weight was seen, as reported for NR1 subunit molecular weight (7).
Using N-hydroxy-succinimide esters as active groups for forming amide bridges with primary amines has been used for ligating antigens to supports and thus purifying antibodies. Several supports have been used, but in our case we used Affi- Gel 10 and Affi-Gel 15. An uncharged spacer was incorporated with Affi-Gel 10 (this being recommended for basic or neutral proteins having 6.5 to 11 isoelectric points). Affi-Gel 15 has a spacer arm having a positively-charged nitrogen (recommended for proteins having isoelectric points less than 6.5) (15).
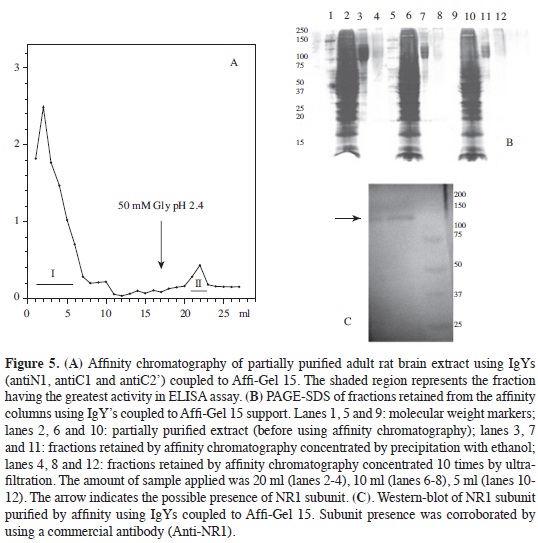
Purifying NR1 subunit Given that IgY did not bind to protein A or protein G, as happens with mammals' immunoglobulins, NR1 subunit was affinity purified with IgY-AffiGel support. NR1 subunit from rat brain extracts (after being incubated with antibody coupled to the support) presented an elution profile (Figure 5A) in which most proteins were eluted in the non-retained fraction whilst the retained fraction (eluted by adding glycine solution, pH 2.4) clearly had less and contained NR1 subunit according to ELISA determination.
Given that protein concentration in retained fractions was very low, they had to be precipitated with absolute ethanol at -20 °C, since concentration by ultrafiltration did not allow the bands corresponding to NR1 subunit to be observed by PAGE-SDS (Figure 5B). Electrophoretic analysis of fractions precipitated with ethanol revealed antibody-retained proteins in the 130 kDa area, agreeing with NR1 subunit molecular weight (no significant percentage of additional bands being observed).
Final confirmation of the presence of NR1 subunit was obtained with immunotransference with the fractions precipitated with ethanol (Figure 5C) where it was seen that NR1 subunit corresponded to the band observed in PAGE-SDS having a molecular weight of around 130 kDa. Additional data of specificity of antibodies produced was determined by immunohistochemistry on rat brain slices; MALDI-TOF MS determinations of affinity-purified NR1 subunit (data not shown) corroborated the purity of the protein. It is worth mentioning that this separation was achieved by using the three antibodies coupled to the same support and that possibly the three antibodies' multiple interaction with NR1 subunit increased efficiency in separation from the subunit due to the great probability that this was a homomeric complex, being corroborated by the single band found in the electrofocusing done on this sample (data not shown).
Regarding the subunit's separation from the receptor, it should be noted that it has been proposed that the different subunits could be separated by using the antibodies produced; however, no description of the separation of any of them has been reported to date. Evidence has been brought forward (21) supporting the presence of a relatively high amount of a pool of unassembled NR1 in brain. NR1 subunit could thus coexist with an assembled homomeric complex or as a simple subunit being ready to bind to NR2 subunits. The fact that particular mRNA from NR1 could produce a functional receptor in oocytes indicates that NR1 subunit could form a homomeric complex receptor in certain conditions, (26, 27). However, a functional homomeric receptor cannot be formed in transfected cells, even though it seems that NR1 subunit could form a complex receptor having binding sites for co-agonist glycine. As unassembled NR1 subunits seem not to be expressed on a neuron's surface (28), then the presence of a pool in excess of NR1 implies that NR2 subunits expression defines the NMDA receptor's functional properties and that NR2 subunits are more finely regulated than NR1 subunits regarding regional expression and development level (28, 29).
ACKNOWLEDGEMENTS
The authors would like to express their thanks to Fundación Instituto de Inmunología de Colombia (FIDIC) for synthesising the peptides, Universidad Nacional de Colombia, Facultad de Medicina Veterinaria y Zootecnia for its support in caring for the animals, Dr Nohora Vega (GRIP, Universidad Nacional de Colombia) for her valuable collaboration and consultancy in purifying the antibodies and Jason Garry for translating the manuscript.
FINANCIAL SUPPORT
This work was supported by Pontificia Universidad Javeriana and the Chemistry Department of Universidad Nacional de Colombia.
REFERENCES
1. Leslie GA, Clem LW. Phylogeny of immunoglobulin structure and function III. Immunoglobulins of the chicken. J Exp Med. 1969. 130: 1337-1352. [ Links ]
2. Larsson A, Karlsson-Parra A, Sjöquist J. Use of chicken antibodies in enzyme immunoassays to avoid interference by rheumatoid factors. Clin Chem. 1991. 37: 411-4. [ Links ]
3. Kronvall G, Seal US, Finstad J, Williams RC. Phylogenetic insight into evolution of mammalian Fc fragment of gG globulin using staphylococcal protein A. J Immunol. 1970. 104: 140-7. [ Links ]
4. Larsson A, Wejaker PE, Forsberg PO, Lindahl T. Chicken antibodies: a tool to avoid interference by complement activation in ELISA. J Immunol Methods.1992. 156: 79-83. [ Links ]
5. Howard GM. Duck immunoglobulins. Structural studies on 5,7 S and 7,8 S gamma globulins. J Immunol. 1967. 98: 811-9. [ Links ]
6. Warr G, Magor K, Higgins D. IgY: clues to the origins of modern antibodies. Immunol Today. 1995. 16: 392-8. [ Links ]
7. Wenthold R, Blahos J, Huh K, Petralia, R. Detergent solubilization and immunoprecipitation of native NMDA receptors. Methods in Molecular Biology. NMDA Receptor Protocols. Humana Press. New Jersey. 1999. 128: 113-9. [ Links ]
8. Houghten RA. Simultaneous multiple peptide synthesis: the rapid preparation of large numbers of discrete peptides for biological, immunological and methodological studies. Bio- Techniques. 1986. 4: 522- 6. [ Links ]
9. Jensenius, JC, Koch C. Antibodies packaged in eggs. In Immunochemistry I. Johnstone A.P., Turner M.W. (eds). IRL Press. Oxford. 1997: 89-107 [ Links ]
10. Gassmann M, Thömmes P, Weiser T, Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990. 4: 2528-32. [ Links ]
11. Akita EM, Nakai S. Immunoglobulins from egg yolk. Isolation and purification. J Food Sci. 1992. 57: 629-34. [ Links ]
12. Barroso P, Murcia H, Vega N, Pérez, G. Obtención y purificación de IgY dirigidas contra la lectina de Salvia bogotensis. Biomédica. 2005. 25: 496-510. [ Links ]
13. Hutchens TW, Porath J. Thiophilic adsorption of immunoglobulinsanalysis of conditions optimal for selective immobilization and purification. Anal Biochem. 1986. 159: 217-26. [ Links ]
14. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227: 680-5. [ Links ]
15. Hermanson G, Mallia K, Smith P. Immobilized affinity ligand techniques. New York. Academic Press Inc. 1992. 110-6. [ Links ]
16. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985. 150: 76-85. [ Links ]
17. Towbin, H., Staehelin, T. and Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979. 76: 4350-4. [ Links ]
18. Méndez, G., Reyes, E., Poutou, R., Quevedo, B., Lareo, L. Purification of IgY against the NR3 subunit of the rat brain NMDA receptor. Rev. MVZ Córdoba. 2008. 13: 1146- 1156 [ Links ]
19. Camenisch G, Tini M, Chilov D, Kvietikova I, Srinivas V, Caro J, Spielmann P, Wengerm R, Gassman M. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1α. FASEB J. 1999. 13: 81-8. [ Links ]
20. Welling GW, Weijer WJ, Van Der Zee R, Welling-Wester S. Prediction of sequential antigenic regions in proteins. FEBS Lett. 1985. 188: 215-8. [ Links ]
21. Blahos J, Wenthold R. Relationship between N-Methyl-D-aspartate receptor NR1 splice variants and NR2 subunits. J Biol Chem. 1996. 271: 15669-74. [ Links ]
22. Hopp T, Woods T. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA. 1981. 78: 3824-8. [ Links ]
23. Kyte J, Doolittle R. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982. 157: 105-32. [ Links ]
24. Hansen P, Scoble J, Hanson B, Hoogenraad N. Isolation and purification of immunoglobulins from chicken eggs using thiophilic interaction chromatography. J Immunol Meth. 1998. 215: 1-7. [ Links ]
25. Porath J, Maisano F, Belew M. Thiophilic adsorption: a new method for protein fractionation. FEBS Lett. 1985. 185: 306-10. [ Links ]
26. Ehlers M, Fung E, O'Brien R, Huganir R. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998. 18: 720-30. [ Links ]
27. Huh K, Wenthold R. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-Methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem. 1999. 274: 151-7 [ Links ]
28. Lau LF, Mammen A, Ehlers MD, Kindler S, Chung WJ, Garner CC, Huganir Rl. Interaction of the NMethyl- D-aspartate receptor complex with a novel synapse-associated protein, SAP102. J Biol Chem. 1996. 271: 21622-8. [ Links ]
29. Petralia R. The ionotropic glutamate receptors. Humana Press, Totowa, NJ. 1997. 219-63. [ Links ]





![EVALUATION OF THE CITOTOXICITY AND ANTIMICROBIAL ACTIVITY OF THE [Ag(phen)2]salH COMPOUND](/img/en/next.gif)