Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.41 no.1 Bogotá Jan./Apr. 2012
EFFECT OF TEMPERATURE AND CONCENTRATION ON THE VISCOSITY OF AQUEOUS SOLUTIONS OF 3-AMINOPROPANOIC ACID, 4-AMINOBUTANOIC ACID, 5-AMINOPENTANOIC ACID,6-AMINOHEXANOIC
ACID EFECTO DE LA TEMPERATURA Y LA CONCENTRACIÓN EN LA VISCOSIDAD DE SOLUCIONES ACUOSAS DE ÁCIDO 3-AMINOPROPANOICO, ÁCIDO 4-AMINOBUTANOICO, ÁCIDO 5-AMINOPENTANOICO, ÁCIDO 6-AMINOHEXANOICO
EFEITO DA TEMPERATURA E A CONCENTRAÇÂO NA VISCOSIDADE DAS SOLUÇÕES AQUOSAS DE ÁCIDO 3-AMINOPROPANOICO, ÁCIDO 4-AMINOBUTANOICO, ÁCIDO 5-AMINOPENTANOICO, ÁCIDO 6-AMINOHEXANOICO
Carmen M. Romero1,2, Alejandro Beltrán1.
1 Universidad Nacional de Colombia, sede Bogotá, Facultad de Ciencias, Departamento de Química, Grupo de Termodinámica Clásica, Laboratorio de Investigaciones Básicas. Av Cra 30 45-03- Bogotá D.C., Código Postal 111321 - Colombia.
Recibido: 09/04/12 – Aceptado: 30/04/12
ABSTRACT
In this work we present the effect of temperature on the viscosities of aqueous so-lutions of 3-aminopropanoic acid, 4-ami-nobutanoic acid, 5-aminopentanoic acid and 6-aminohexanoic acid as a function of concentration. The experimental mea-surements were done from 293.15 K to 308.15 K. At each temperature the experimental data were fitted to the Tsangaris-Martin equation and the B viscosity coefficient was determined. The dependence of the B coefficients on the number of carbon atoms of the amino acids is linear, so the contribution of polar and apolar groups was established. The results are interpreted in terms of amino acid hydration.
Key words: Amino acid; viscosity; hydrophobic hydration; solute-solvent interactions.
RESUMEN
En este trabajo se presenta el efecto de la temperatura sobre las viscosidades de soluciones acuosas de acido 3-aminopro-panoico, acido 4-aminobutanoico, acido 5-aminopentanoico, acido 6-aminohexa-noico en función de la concentración. Las determinaciones experimentales se realizaron en un intervalo de temperatura entre 293,15 K hasta 308,15 K. A cada una de las temperaturas trabajadas, los datos experimentales se ajustaron a la ecuación de Tsangaris-Martin y se determinó el coeficiente B de viscosidad. La dependencia del coeficiente B con el número de carbonos del aminoácido es lineal lo cual permitió establecer la contribución de los grupos polares y apo-lares del aminoácido. Los resultados se interpretan en términos de la hidratación de los aminoácidos.
Palabras clave: aminoácidos; viscosidad; hidratación hidrofóbica; interacción soluto-solvente.
RESUMO
Neste trabalho é apresentado o efeito da temperatura sobre as viscosidades das soluções aquosas de ácido 3-aminopro-panoico, ácido 4-aminobutanoico, ácido 5-aminopentanoico, ácido 6-aminohe-xanoico em função da concentração. As determinações experimentais foram feitas em um intervalo de temperatura entre os 293,15 K até 308,15 K. Para cada uma das temperaturas trabalhadas, os dados experimentais foram ajustados à equação de Tsangaris-Martin, e o coe-fciente B de viscosidade foi determinado. A dependência do coeficiente B com o número de carbonos do aminoácido é linear e permitiu estabelecer a contri-buição dos grupos polares e apolares do aminoácido. Os resultados foram interpretados em termos da hidratação dos aminoácidos.
Palavras-chave: aminoácidos; vis-cosidade; hidratacao hidrofóbica; intera-cao soluto-solvente.
INTRODUCTION
Amino acids have been considered model compounds that can give useful information to understand the behavior of proteins and the role of solvent struc-ture in the denaturation process. For this reason and due to their applications, the properties of amino acids in aque-ous solution including their viscometric properties have been extensively studied (1-18). The information has been used to provide an insight about hydropho-bicity, hydration properties and solute-solvent interactions. However most of these studies refer to naturally occurring small a-amino acids at 298.15 K and very few data are reported in literature for α,ω-amino acids specially at tem-peratures different from 298.15 K (5, 8, 15, 16).
Although there is much literature on aqueous solutions of a-amino acids, data on the viscosities of solutions of a,co-amino acids are limited in particular at temperatures different from 298.15 K. In order to extend the understanding on the behavior of aqueous solutions of amino acids and as continuation of our previous experimental studies on viscosity of the aqueous mixtures of these solutes (11, 13, 14), we report here new experimental viscosity data for aqueous solutions of 3-amino propanoic acid, 4-amino bu-tanoic acid, 5-amino pentanoic acid and 6-amino hexanoic acid as a function of concentration at 293.15, 298.15, 303.15 and 308.15 K. The amino acids were chosen to examine the effect of increase in the number of CH. groups.
The behavior of the viscosity of solutions is a consequence of the different interactions between solute and the solvent molecules. For this reason it depends on the nature of solvent and solute nature, the size and shape of the latter, concentration, and is affected by external factors such as temperature. Several industrial applications are very sensitive to flow behavior and are specially affected by the behavior of viscosity with temperature. In this regard, information about the flow properties of aqueous solutions of a,co-amino acids is important. Of particular interest is the behavior of the relative viscosity as a function of concentration, since it gives important information about solute hydration.
In this work the relative viscosity results were used to determine the fi-coefficients of amino acids at the selected temperatures. The dependence of the B coefficient with temperature was used to discuss the effect of the hydrocarbon chain on water structure and the hydration properties of the solutes. A comparison between the B coefficients of α-amino acids and α,ω-amino acids is presented. The results show that the coefficients for a,α-amino acids are smaller than the corresponding valúes for Α-amino acids at the same temperatures suggesting that solute-solvent hydrophobic interactions are larger for aΑ-amino acids due to the more exposed aliphatic chain to the solvent.
MATERIALS AND METHODS
The materials used in this work were: 3-aminopropanoic acid ((β-alanine) Merck purity ≥ 99 %, 4-aminobuta-noic acid (γ-aminobutyric acid) Merck purity ≥ 98 %, 5-aminopentanoic acid (δ-aminovaleric acid) Aldrich purity ≥ 97 % and 6-aminohexanoic acid (ε-aminocaproic acid) Merck purity ≥ 99 %. The amino acids were dried under vacuum at room temperature and kept in desiccator at least 48 h before use. Water was doubly distilled and deionized ac-cording to literature and degassed before use obtaining a product with conductiv-ity less than 2 µS m ' (19, 20). All solutions were prepared by weight using a Mettler balance AT 261 dual range with sensitivity of ÍO5 g in the lower range.
Viscosity n was determined using two Ubbelohde viscometers with efflux times t near 500 s for water that were cal-ibrated with water at the selected temperatures. Reproducibility of efflux times was in all cases better than 0.05 %. Efflux times were determined at 293.15,298.15, 303.15 and 308.15 K for the amino acid aqueous solutions. All measurements were carried out in a constant tempera-ture bath with temperature controlled to + 0.005 K. Reported valúes are the av-erage of at least three independent measurements in each viscometer. Densities ρ of the solutions at the selected temperatures were taken from a previous work (21). They were measured at the same temperatures using pycnometers of the Wood-Brusie type, in a constant temperature bath with temperature controlled to ± 0.01 K. Density valúes are the average of three independent measurements and uncertainty in the density measurements is estimated to be + 5-105 g cm3
The viscosity data were obtained from the general equation:
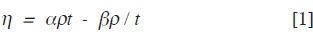
125
where α and β are the viscometer con-stants obtained by calibration with water at each temperature, ρ is the density and t the efflux time. The relative viscosities nr= n/no were calculated from the solu-tion and solvent viscosities respectively.
RESULTS AND DISCUSSION
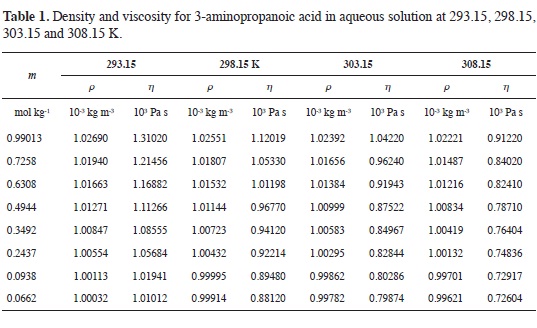
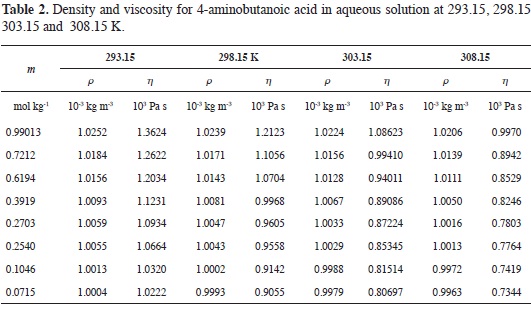
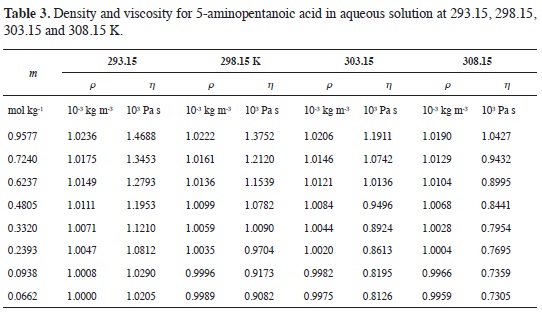
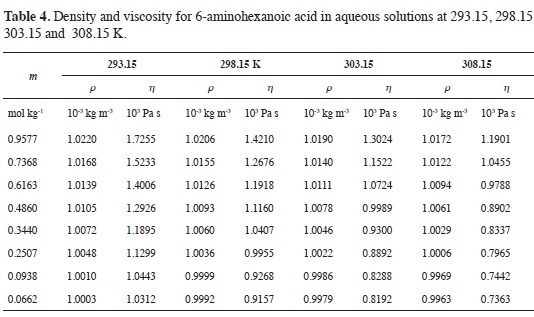
Tables 1-4 show the experimental results for density and viscosity of the aque-ous solutions of 3-aminopropanoic acid, 4-aminobutanoic acid, 5-aminopenta-noic acid, and 6-aminohexanoic acid as a function of molality at 293.15, 298.15, 303.15 and 308.15 K. The density data were obtained adjusting the valúes re-ported in literature from a previous work at the same temperatures considered in this work (21).
The concentration dependence of the viscosity of aqueous solutions of α,ω-amino acids exhibits the usual behavior.
At all temperatures, solution viscosity increases with amino acid concentration and the effect is more pronounced as the length of the hydrocarbon chain becomes larger. From Tables 1-4 it can be seen that for each amino acid, the viscosity decreases as temperature increases.
The relative viscosity valúes were calculated and adjusted by least-squares to a second order equation as proposed by Tsangaris-Martin (3).
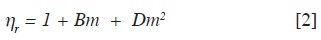
In this equation B and D are empirical coefficients; however it has been sug-gested that the B coefficient depends on the size, shape and charge of the solute molecule and is sensitive to solute solvent interactions (4, 15-18). The effect of temperature on the viscosity of the sol-utes in aqueous solution is well described by a polynomial second order equation. Table 5 shows the valúes of the B coefficients of α,ω-amino acids in aqueous solution obtained in this work at 293.15, 298.15, 303.15 and 308.15 K.
α,ω-amino acids in aqueous solution show relatively large positive B coefficients that increase with the number of CH. groups. This behavior indicates that the magnitude of the coefficients is a consequence of the increase in the hy-drophobic character of the amino acid as the hydrocarbon chain length increases.
Even though the effect of temperature is small, the observed trend shows a de crease of the coefficients with tempera-ture. The first derivative of the B coefficient with temperature áBláT has been used as indicative of the effect of solute on water structure (16-18). The negative sign has been attributed to a structure making effect while the positive sign to a structure breaking effect on water structure. In this study, all the solutes consid-ered, shows a negative valué for áBláT suggesting that 3-aminopropanoic acid, 4-aminobutanoic acid, 5-aminopentanoic acid and 6-aminohexanoic acid have a water structure making character.
Table 6 presents a comparison be-tween the B coefficients of α-amino ac-ids and α,ω-amino acids including litera-ture data.
For glycine, the coefficient increases with temperature, as has been reported by several authors who explain this be-havior as a consequence of the breaking structure effect that this solute exerts on water structure (2,4, 5, 16). For the other amino acids, the behavior changes, the coefficient decreases with temperature and áBláT shows a negative valué at all temperatures suggesting that they have a water structure making character. The calculated valúes are small and do not follow a definite trend. Taking into ac-count the experimental uncertainty, they are not reported and the discussion is re-ferred only to the sign of the slope.
It is clear that in aqueous solutions, the coefficients for α,ω-amino acids are smaller than the corresponding valúes for α-amino acids at the same temperatures as is shown in Table 6. This suggests that solute-solvent hydrophobic interactions are larger for α-amino acids due to the more exposed aliphatic chain to the solvent (21).
At each temperature the B coefficient is a linear function of the number of carbón atoms of the hydrocarbon chain. These results show that it can be described in terms of the contributions of the charged and methylene groups, ac-cording to a simple additivity approach. This simple model is based on the depen-dence of the property on the number of carbón atoms or the number of methylene groups (11, 18, 22, 23), and can be represented by the following equation:
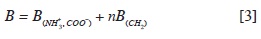
Table 7 shows the behavior of the vis-cometric group contributions to the B coefficient with temperature. The observed change is very small and the general trend is to diminish as temperature in creases, following the observed behavior reported in literature for a-amino acids (11, 16).
ACKNOWLEDGMENTS
This work was supported by the DIB Grant 13007 from Universidad Nacional de Colombia.
REFERENCES
1. Belibagli, K. B.; Ayranci, E. Visco-sities and apparent molar volumes of some amino acids in water and in 6 M guanidine hydrochloride at 25 °C. /. Solution Chem. 1990. 19 (9): 867-882. [ Links ]
2. Ali, A.; Hyder, S.; Sabir, S.; Chand, D.; Nain, A. K. Volumetric, vis-cometric, and refractive Índex be-haviour of a-amino acids and their groups' contribution in aqueous D-glucose solution at different temper-atures. /. Chem. Thermodyn. 2006. 38 (2): 136-143. [ Links ]
3. Tsangaris, J. M.; Martín, R. B. Vis-cosities of aqueous solutions of di polar ions. Arch. Biochem. Biophys. 1965. 112 (2): 267-272. [ Links ]
4. Tyrrell, H. J. V.; Kennerley, M. Vis-cosity B-coefficients between 5 °C and 20 °C for glycolamide glycine, and n-mefhylated glycines in aqueous solution, J. Chem. Soc., A: Inorg. Phys. Theor. 1968.1968: 2724-2728. [ Links ]
5. Devine, W.; Lowe, B. M. Viscosity B-coefficients at 15 and 25 °C for glycine, (3-alanine, 4-amino-n-butyr-ic acid, and 6-amino-n-hexanoic acid in aqueous solution, /. Chem. Soc, A: Inorg. Phys. Theor. 1971. 1971: 2113-2116. [ Links ]
6. Masón, L. S.; Kampmeyer, P. M.; Robinson, A. L. The viscosities of aqueous solutions of amino acids at 25 °C and 35 °C. /. Am. Chem. Soc. 1952.74(5): 1287-1290 [ Links ]
7. Awasthi, O. R; Rastogi, R P. Viscosity B-coefficient of some amino acids in water. /. Iridian Chem. Soc. 1980. 57: 1204-1207. [ Links ]
8. Daniel, J.; Cohn, E. J. Studies in the physical chemistry of amino acids, peptides and related substances: VI. The densities and viscosities of aqueous solutions of amino acids. /. Am. Chem. Soc. 1936. 58 (3): 415-423. [ Links ]
9. Yan, Z.;Wang, J.; Liu, W.; Lu, J. Apparent molar volumes and viscosity B coefficients of some a-amino acids in aqueous solutions from 278.15 K to 308.15 K. Thermochim. Acta. 1999.334(1-2) 17-27. [ Links ]
10. Cohn, E. J.; Edsall, J. T. Proteins, amino acids and peptides as ions and dipolar ions. New York, Hafner Pub-lishing Company. 1965. [ Links ]
11. Romero, C. M.; Negrete, F. Effect of temperature on partial molar voluntes and viscosities of aqueous solutions of a-DL-aminobutyric acid, DL-norvaline and DL-norleucine. Phys. Chem. Liq. 2004. 42 (3): 261-267. [ Links ]
12. Sandu, J. S.; Kashyap, U. The viscosities of aqueous solutions of some amino acids. Electrochem. Soc. India. 1986. 35: 283-287. [ Links ]
13. Romero, C. M.; Moreno, E.; Rojas, J. L. Apparent molar volumes and viscosities of a-DL-alanine in water-alcohol mixtures. Thermochim. Acta. 1999. 328 (1-2): 33-38. [ Links ]
14. Romero, C. M; Rojas, J. L. Apparent molar volumes and viscosities of a-Dl-alanine in water-efhanol mixtures. Phys. Chem. Liq. 2000. 38 (2): 237-243. [ Links ]
15. M. Natarajan, R. K. Wadi, H. C. Gaur, Apparent molar volumes and viscosities of some a- and a,co-amino acids in aqueous ammonium chloride solutions at 298.15 K. /. Chem. Eng. Data. 1990. 35 (1): 87-93. [ Links ]
16. Zhao, H. Viscosity B-coefficients and standard partial molar volumes of amino acids, and their roles in interpreting the protein (enzyme) stabilization. Biophys. Chem. 2006. 122 (3): 157-183. [ Links ]
17. Lark, B. S.; Patyar, P.; Tarlok, S. B. Temperature effect on the viscosity and heat capadty behaviour of some amino acids in water and aqueous magnesium chloride solutions. /. Chem. Thermodyn. 2007. 39 (3): 344-360. [ Links ]
18. Banipal, T. S.; Gagandeep, S. Ther-modynamic study of solvation of some amino acids, diglycine and lysozyme in aqueous and mixed aqueous solutions. Thermochim. Acta. 2004. 412 (1-2): 63-83. [ Links ]
19. Riddick, J. A.; Bunger, W. B.; Sakano, T.K. Organic solvents: physical properties and methods of purification. New York, Wiley-Interscience. 1986. [ Links ]
20. Weisseberger, A.; Rossiter, B. W. Physical methods of chemistry New York, Wiley-Interscience. 1972. Vol. 4. [ Links ]
21. Romero, C. M; Cadena, J.C. Effect of temperature on the volumetric properties of a,co-amino acids in dilute aqueous solutions. /. Solution. Chem. 2010. 39 (10): 1474-1483. [ Links ]
22. Hakin, A. W.; Duke, M. M; Groft, L. L.; Marty, J. L.; Rushfeldt, M. L. Calorimetric investigations of aqueous amino acid and dipeptide systems from 288.15 K to 328.15 K. Can.]. Chem. 1995. 73 (5): 725-734. [ Links ]
23. Hakin, A. W.; Copeland, A. K.; Liu, J. L.; Marriott, R. A,; Preuss, K. E. Densities, apparent molar volumes, and apparent molar heat capacities of 1-arginine, 1-proline and 1-methio-nine in water at 288.15, 298.15, 313.15, and 328.15 K. /. Chem. Eng. Data. 1997. 42 (1) 84-89. [ Links ]