Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.41 no.3 Bogotá Sept./Dec. 2012
Aroylhydrazones as potential systems for information storage: photoisomerization and metal complexation
Las aroilhidrazonas como sistemas potenciales para el almacenamiento de información: fotoisomerización y complejación de metales.
Aroylhydrazones como sistemas potenciais para o armazenamento de informação: fotoisomerização e complexação de metais
Manuel N. Chaur*
* Departamento de Química, Universidad del Valle, 25360 Santiago de Cali, Colombia. manuel.chaur@correounivalle.edu.co
Recibido: 08/10/12 Aceptado: 13/12/12
ABSTRACT
Aroylhydrazones are compounds formed from the condensation of an acylhydrazine and an aldehyde. These compounds exhibit dynamic reversible properties such as isomerization photochemically and thermally activated, hydrazine substitution and coordination to metallic centers. All these together represent systems with multiple dynamics suitable for information storage devices and for the design of molecular photoswitches.
Key words: aroylhydrazones, storage, photoisomerization, metal complexation.
RESUMEN
Las aroilhidrazonas son compuestos formados a partir de la condensación de una acilhidrazina y un aldehído. Estos compuestos presentan propiedades dinámicas reversibles tales como la isomerización activada térmica y fotoquímicamente, la sustitución de hidracina y la coordinación con centros metálicos. Todas estas representan sistemas con dinámicas múltiples apropiadas para dispositivos de almacenamiento de información y para el diseño de foto-interruptores moleculares.
Palabras clave: Aroilhidrazonas, almacenamiento,fotoisomerización, complejación de metales.
RESUMO
Aroilhidrazonas são compostos formados a partir da condensação de uma acilhidrazina e um aldeído. Estes compostos apresentam propriedades reversíveis dinâmicas, como isomerização fotoquímica e termicamente ativada, substituição de hidrazina e coordenação de centros metálicos. Todos estes em conjunto representam sistemas com múl350 Revista Colombiana de Química, Volumen 41, nro. 3 de 2012 tiplas dinâmicas adequadas para dispositivos de armazenamento de informações e para o desenho de fotodispositivos moleculares.
Palavras-chave: Aroilhidrazonas,armazenamento, fotoisomerização, complexação de metais.
INTRODUCTION
Aroylhydrazones, oximes, hydrazones and, in general, imines exhibit very interesting properties such as configurational dynamics arising from their E/Z isomerization photochemically and thermally induced. (1,2) It has been conjectured, that this feature provides imines with the attributes of unidirectional photoactivated molecular motors, (2) as they proceed through different isomerization pathways, photoinduced out-of-plane rotation about the C=N bond and thermally- activated in-plane nitrogen inversion. (1) The latter play a major role in constitutional dynamic processes (3) of the covalent type. (4)
Other feature is the ability of going through an exchange of the carbonyl or amine group by a transimination reaction, which is called constitutional dynamics.(3) Of crucial interest for the purpose of this paper is the fact that the configurational isomerization occurs with conservation of the constitutional integrity of the substance and is comparatively fast (or may be made so), whereas component exchange generates a new molecule at a rate that may be chemically controlled and is usually much longer than configurational isomerization (Scheme 1). In other words, configurational and constitutional changes are comparatively fast and slow processes, so that iminetype entities allow for both short-lived and long-lived information storage, respectively in the configuration and in the constitution of a molecule. Short term storage is structurally/physically borne by a molecular shape, and long term storage is inscribed in the formation of a novel molecule. Importantly, both processes are orthogonal and may be separately controlled/operated. Such features are reminiscent of short term memory/information storage in a molecular conformation and of long term memory/information storage in the synthesis of a novel molecule. The latter may be made irreversible/permanent by performing a reaction on the C=N bond (e.g. reduction, addition).(5)
Another important process is the ability of certain aroylhydrazones, and hydrazones containing pyridine groups, of coordinating to metal centers. (4-8) Although coordination of the NNN site of bis-pyridylhydrazones to metals is stronger, complexes of aroylhydrazones contain a more acidic amide proton. As a result, the deprotonation yields a monoanionic ligand able to bind to metal ions more efficiently. The scheme 1 shows the multiple dynamics of these compounds.
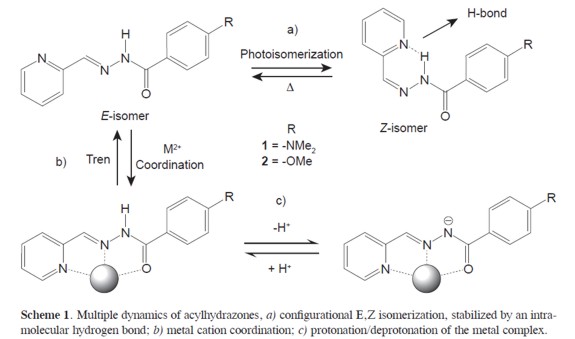
In the present work were prepared two aroylhydrazones which exhibit the aforementioned features acting as compounds of multiple dynamics able of going through different metastable states in a reversible fashion and as a result these compounds are suitable for information storage and for the design of molecular photoswitches. Herein are describeda set of processes that embody the aforementioned features and may be considered as prototypes stimulating further development and suggesting perspectives in the search for multiplex chemical (molecular and supramolecular) information storage and processing devices, in view of i) their response to multiple stimuli of physical (photo and thermo) as well as chemical nature, ii) their ability to generate constitutional variation by component exchange, and iii) their kinetic properties, involving very different but controllable interconversion rates between the various configurational and constitutional states. The hydrazine exchange properties have been studied before (3-5) thus, focus is made on the kinetics of photoisomerization and thermal-back conversion as wells as the optical properties of the metastable states. Finally,the molecular structure of a metal complex of ZnII is presented with one of the acylhydrazones reported here as obtained from single crystal X-Ray diffraction.
RESULTS AND DISCUSSION
Photoisomerization of aroylhydrazones is accelerated and stabilized by an intramolecular hydrogen bond between the amide proton and the nitrogen of the pyridine ring. (1, 9) The formation of such H-bond increases the thermodynamic stability of the Z isomer. Therefore the speed of isomerization and thermal-back conversion is increased by an increase in the strength of the H-bond (see Figures 1-2). For instance, compound 2-Z is favored, when compared with compound 1-Z, by the more electron-withdrawing group (-OMe).Compound 2-E exhibits a larger rate of isomerization (k=0.086 min-1) than compound 1-E (k=0.012 min-1) which is evident from their t1/2 of 8.0 and 54.5 minutes respectively. The hydrazone counterpart, the 2-pyridinecarbaldehyde phenylhydrazone (3-Z), follows similar isomerization kinetics with k=0.019 min-1 and t1/2=36.4 min.
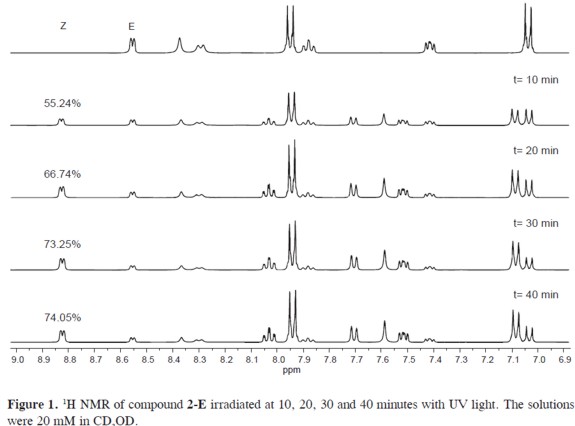
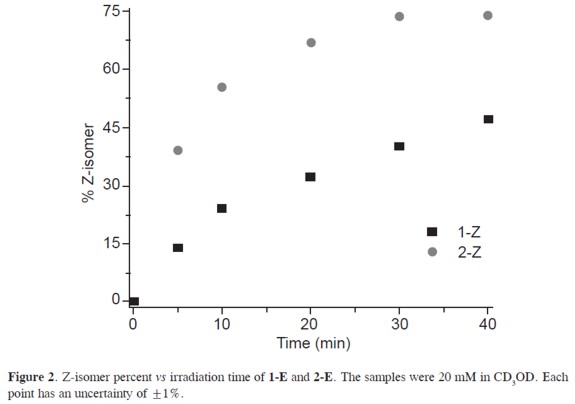
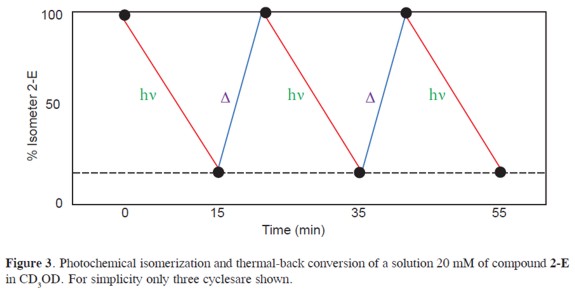
Return to the E state is accomplished by thermal-back isomerization. This occurs under 5 minutes of reflux or by heating (5 minutes, 40 °C) accompanied with addition of catalytic amounts of trifluoroacetic acid (see Figure 3). The process of photoisomerization (E→Z conversion) and thermal-back isomerization (Z→E conversion) can be implemented during several cycles without decomposition of the constituents.
Previous photochemical studies of hydrazones have shown that direct excitation leads to E,Z isomerization via the singlet state and a rapid relaxation to the minimum of the potential energy surface where it acquires an intermediate geometry between the syn and anti configurations, this is also true for aroylhydrazones. This isomerization is independent of concentration and wavelength. The UV-vis spectrum (see Figure 4) shows a red shift in the absorption wavelength as going from the E to the Z forms, besides the Z compound exhibits a decrease in the absorption of about 20%.
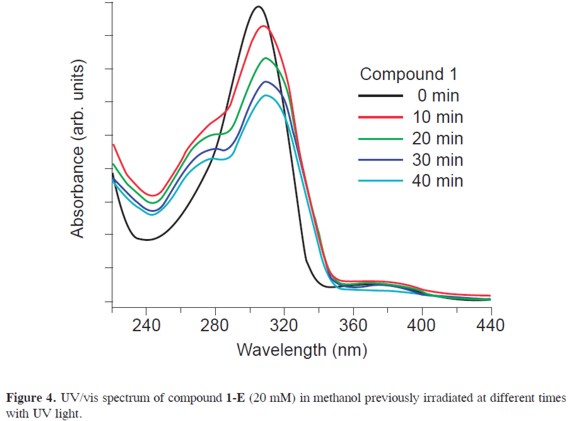
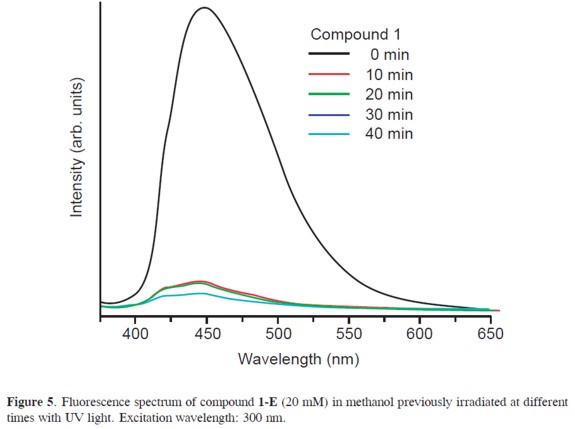
Additionally, these compounds exhibit different fluorescence properties and a shift of the optical absorptions as they go from E to Z configurations. Compound 1-Z exhibits a quantum yield, Φfluo, of 0.03 while compound 1-E has a Φfluo∼0. Thus, aroylhydrazones feature the ability of tuning their emission ON and OFF as the molecule undergoes light-induced isomerization (see Figure 5). Intramolecular H-bonding probably causes the fluorescence quenching in this system as it has been observed for other heterocyclic and pyridine based systems.(10)
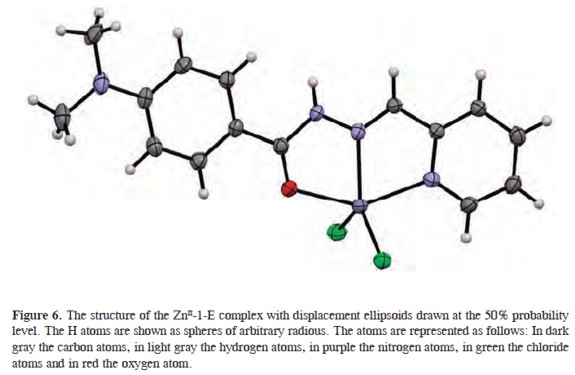
Besides the photochemical properties of these compounds, they also exhibit the ability to coordinate to metal centers. Figure 6 shows the chemical structure of the metal complex formed by the reaction of 1-E with ZnCl2 as determined by X-ray diffraction.
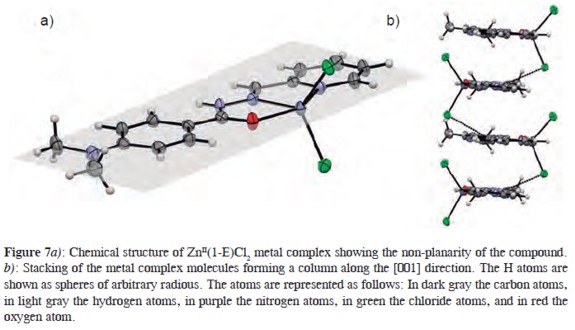
The complex compound exhibits a distorted five-coordinated square-pyramidal disposition. The aroylhydrazone ligand is not planar, resulting in a dihedral angle between the planes of the aromatic and pyridyl rings of 4.61(9)° (see Figure 7a). The compound molecules stack forming columns along the [001] direction by intermolecular hydrogen bonds with a distance N3-H3···Cl2 = 3.199 (2) Å (see Figure 7b). Also it is observed a weak π-π slipped stacking interaction between the aromatic rings with distance centroid-centroid = 3.8075 (1) Å.
Additionally, aroylhydrazones can be deprotonated (amide proton) to yield a monoanionic ligand able to bind a metal ion more efficiently (see scheme 1). All the processes presented herein are reversible(isomerization, coordination, and deprotonation), and can be interchanged along many cycles without decomposition of the system (see Scheme 1 and Figure 3). Thus, the C=N bond of acylhydrazones represents an appealing unit that can be implemented for double dynamic processes of interest for information- storage processes. Its configurational dynamics gives access to short term photoactivated metastable states. Finally, the system can be locked or unlocked as desired by the coordination or removal of a suitable metal ion.
CONCLUSIONS
In conclusion, aroylhydrazones represent a class of compounds that exhibit multiple reversible dynamics (configurational, constitutional and coordination). These features can be implemented in the design of information storage devices since configuration changes are intrinsically short termed, configurational changes are long-termed and coordination locks the system. (10-11) Likewise, these compounds can be used in the design of molecular photo-switches due to the quenching in fluorescence after isomerization. Further work is devoted to the design of such memory devices by the implementation of more complex systems and in the rational design of molecular photo-switches. Finally the aroylhydrazones can be further extended by the substitution on the phenyl and pyridine rings.
ACKNOWLEDGMENTS
This work was supported partly by the Department of Chemistry. El Centro de Excelencia en Nuevos Materiales (CENM) and the vicerrectoría de investigaciones of the Universidad del Valle. The author is also grateful to the service de cristallographie of the Université de Strasbourg.
EXPERIMENTAL PART
The starting materials were purchased from Sigma-Aldrich and used without any further purification. 1H and 13C NMR spectra were taken in a 400 MHz Bruker Ultra Shield spectrometer. UVvis spectra were recorded in a UV-1700 PharmaSpec spectrophotometer. Fluorescence experiments were carried out in a FP-8500 Jascos pectrofluorometer. Fluorescence quantum yields, Φfluo,were measured for all the solutions using quinine sulphate in 0.1 M H2SO4 (Φfluo =0.546) as the standard.
Standard procedure for the preparation of aroylhydrazones: 2-pyridinecarboxaldehyde (1.0 equiv) was added to an ethanol solution of the corresponding aroylhydrazine (1.0 equiv) and a trace amount of glacial acetic acid. After the reaction mixture was heated under reflux for 3.0 h, the precipitates were collected on a Büchner funnel. The aroylhydrazones, which were obtained in 87-90 % yield, were recrystallized from ethanol. The E-isomer was exclusively obtained.
Irradiation procedure. Preparation in the Z formsIrradiations were carried out in a UV Curing Light Source Model 38125 with a 400W lamp UVA (from within approximately 400 to 320nm of the spectral band), metal halide with radiant energy (intensity) 100mW/cm2 at 365nm.
(E)-4-(dimethylamino)-N'-(pyridin- 2-ylmethylene)benzohydrazide (1-E) The product was synthesized using the method described above and obtained in a 90% yield. Melting point 225-226 °C. δH (400 MHz, MeOD) 8.59 – 8.52 (1 H, m), 8.40 – 8.25 (2 H, m), 7.93 – 7.83 (3 H, m), 7.42 (1 H, ddd, J 7.5, 5.0, 1.2), 6.83 – 6.74 (2 H, m), 3.05 (6 H, s). 13C NMR (101 MHz, MeOD) δ = 154.90, 154.88, 150.03, 147.71, 147.69, 138.57, 130.62, 125.73, 122.15, 119.50, 112.12, 40.16. MS (ESI): 269.139 m/z. Elemental analysis of C15H18N4O2 (8-E,H20): C 62.87%, H 6.29%, N 19.46%. Calculated: C 62.92%, H 6.34%, N 19.57%.
(E)-4-methoxy-N'-(pyridin-2-ylmethylene) benzohydrazide (2-E). The product was synthesized using the method described above and obtained in a 87% yield. Melting point 130-131 oC. δH (400 MHz, MeOD) 8.56 (1 H, ddd, J 4.9, 1.5, 0.9), 8.38 (1 H, s), 8.30 (1 H, d, J 7.8), 8.03 – 7.83 (3 H, m), 7.42 (1 H,ddd, J 7.5, 5.0, 1.0), 7.11 – 6.99 (2 H, m), 4.86 (3 H, s). 13C NMR (101 MHz, MeOD) δ = 166.81, 164.68, 154.68, 150.11, 148.75, 138.57, 130.93, 125.89, 125.74, 122.22, 115.00, 56.02. MS (ESI): 256.275 m/z. Elemental analysis C14H13N3O2 C 65.59%, H 5.02%, N 16.32%. Calculated: C 65.87%, H 5.13%, N 16.46%.
REFERENCES
1. Lehn, J. M. Chem. Eur. J. 2006, 12, 5910. [ Links ]
2. Padwa, A. Chem. Rev. 1977, 77, 37; Pratt, A. C. Chem. Soc. Rev. 1977, 6, 63-81; Maeda, K.; Fischer, E. Helv. Chim. Acta. 1983, 66, 1961; Becker, R. S.; Chagneau, F. J. Am. Chem. Soc. 1992, 114, 1373; Kawamura, Y.; Takayama, R.; Nishiuchi, M.; Tsukayama, M. Tetrahedron Letters. 2000, 41, 8101; Suginome, H.; in CRC Hand-book of Organic Photochemistry and Photobiology (Eds.: W. Horspool, F. Lenci), CRC Press LLC, Boca Raton, Fla, 2004, 94, 1 55; Arai, T.; Furuya, Y.; Tokumaru, K. J. Photochem. Photobiol. A: Chemistry. 1996, 97, 133, and references therein. [ Links ]
3. Kandel, E. R. Science. 2001, 294, 1030-1038; Kandel, E. R. Nobel Lectures, Physiology or Medicine 1996-2000, (Ed. H. Jörnvall) World Scientific Publ., 2003, p. 392-439; Barco, A.; Bailey, C. H.; Kandel, E. R. J. Neurochem. 2006, 97, 1520-1533. [ Links ]
4. Choudhury, A., Geetha, B., Sangeetha, N. R., Kavita, V.; Susila, V.; Pal, S. J. Coord. Chem., 1999, 48, 87-95. [ Links ]
5. Choudhary, S.; Morrow, J. R. Angew. Chem. Int. Ed. 2002, 41, 4096-4098. [ Links ]
6. Bernhardt, P. V.; Chin, P.; Sharpe, P. C.; Richardson, D. R. Dalton Trans., 2007, 3232-3244. [ Links ]
7. Chow, C. F.; Fujii, S.; Lehn, J. M. Angew. Chem. Int. Ed. 2007, 46, 5007-5010. [ Links ]
8. Cao, X. Y.; Harrowfield, J.; Nitschke, J.; Ramirez, J.; Stadler, A. M.; Kyritsakas-Gruber, N.; Madalan, A.; Rissanen, K.; Russo, L.; Vaughan, G.; Lehn, J. M. Eur. J. Inorg. Chem. 2007, 2944-2965. [ Links ]
9. Palla, G.; Mangia, A.; Predieri, G. Annali Chim. 1984, 74, 153; Belov, D. G.; Rogachev, B. G.; Tkachenko, L. I.; Smirnov, V. A.; Aldoshin, S. M. Russian Chem. Bulletin. 2000, 49, 666; Syakaev, V. V.; Podyachev, S. N.; Buzykin, B. I.; Latypov, S. K.; Habicher, W. D.; Konovalov, A. I. J. of Mol. Struct. 52006, 788, 55-62. [ Links ]
10. Lehnn J. M.; Chem Soc. Rev. 2007, 36, 151; Chaur, M. N.; Collado, D.; Lehn, J. M. Chem. Eur. J. 2011, 17, 248-258. [ Links ]
11. Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. Vogels´ Textbook of Practical Organic Chemistry. 5th ed.; Longman Scientific and Technical: Harlow, 1989; p. 1258; Butler, R. N.; Johnston, S. M., J. Chem. Soc. Perkin Trans I 1984, 2109-2116; RuHwu, J.; Lin, C. C.; Chuang, S. H.; King, K. Y.; Suc, T. R.; Tsayd S.C., Bioor. Med. Chem. 2004, 12, 2509-2515; Todeschinia, A. R.; Miranda, A. E.; da Silva, K. C. M.; Parrinib, S. C.; Barreiro, E. J. Eur. J. Med. Chem. 1998, 33, 189-199. [ Links ]
12. Chaur, M. N. Acta Cryst. 2013,E69, m27. [ Links ]













