Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.41 no.3 Bogotá Sept./Dec. 2012
Solubility of acetaminophen in polyethylene glycol400 + water mixtures according to the extended Hildebrand
Estimación de la solubilidad del acetaminofeno en mezclas polietilenglicol 400 + agua según el método extendido de Hildebrand
Solubilidade estimada do paracetamol em misturas polietileno glicol 400 + água de acordo com o método estendido de Hildebrand
Edgar A. Ahumada1, Daniel R. Delgado1, Fleming Martínez1*
1Group of pharmaceutical-physicochemical research, Department of Pharmacy, Universidad Nacional de Colombia,PO Box 14490, Bogotá, D. C., Colombia.
*Correspondence concerning this paper should be addressed to Fleming Martínez, Department of Pharmacy, Universidad Nacional de Colombia. fmartinezr@unal.edu.co
Recibido: 20/08/12 – Aceptado: 27/11/12
Abstract
The Extended Hildebrand Solubility Approach(EHSA) was applied in the present work to evaluate the solubility of the analgesic drug acetaminophen (paracetamol) in polyethylene glycol 400 + water mixtures at 298.15 K. An acceptable correlative capacity of EHSA was found using a regular polynomial model in order four (overall deviation below 0.7%), when the W interaction parameter is related to the solubility parameter of the mixtures. Thus, the deviations obtained in the estimated solubility with respect to experimental solubility were lower than those obtained directly by means of an empiric regression of the experimental solubility as a function of the mixtures´ solubility parameters (close to 1.5%).
Key words: acetaminophen, binary mixtures, extended Hildebrand solubility approach, solubility parameter.
Resumen
En el presente trabajo se aplicó el Método Extendido de Solubilidad de Hildebrand (MESH) al estudio de la solubilidad del acetaminofeno en mezclas binarias polietilenglicol 400 + agua a 298,15 K. Se obtuvo una capacidad predictiva aceptable del MESH (desviación general inferior al 0,7%) al utilizar un modelo polinómico regular de cuarto orden que relaciona el parámetro de interacción W con el parámetro de solubilidad de las mezclas solventes. Las desviaciones obtenidas en la solubilidad estimada fueron de menor magnitud que las obtenidas al calcular esta propiedad directamente, utilizando una regresión empírica regular del mismo orden de la solubilidad experimental del fármaco en función del parámetro de solubilidad de las mezclas disolventes (cerca del 1.5%).
Palabras clave: acetaminofeno, Método Extendido de Solubilidad de Hildebrand, mezclas binarias, parámetro de solubilidad.
Resumo
O método estendido de solubilidade de Hildebrand (MESH) foi aplicado nesta pesquisa para avaliar a solubilidade do paracetamol em água de misturas binárias + polietileno glicol 400 em 298,15 K. Obteve-se boa capacidade preditiva com o MESH (desvio inferior a 0,7%) quando se utiliza um polinômio regular de quarta ordem do parâmetro de interação W com o parâmetro de solubilidade das misturas de solventes. Os desvios obtidos na solubilidade estimada foram inferiores do que os obtidos através do cálculo desta propriedade diretamente, utilizando uma regressão normal empírica da mesma ordem da solubilidade experimental da droga em função do parâmetro de solubilidade das misturas solventes (cerca de 1,5 %).
Palavras-chave: acetaminofeno, Método estendido de solubilidade de Hildebrand, misturas binárias, parâmetro de solubilidade.
INTRODUCTION
Acetaminophen (ACP, Figure 1) is a drug widely used as analgesic and antipyretic which physicochemical properties have not yet been studied throroughly (1). In particular, its solubility in aqueous media is very important in several processes associated to research and development during the design of homogeneous liquid dosage forms intended mainly for pediatric patients (2). It is important to note that cosolvency is the best technique used in pharmacy to increase drug solubility (3). On the other hand, it is clear that predictive methods of physicochemical properties of drugs, in particular its solubility, are very important for industrial pharmacists because they allow the optimization of design processes (4).
For this reason, the present work presents a physicochemical study about the solubility prediction of ACP in binary mixtures conformed by polyethylene glycol 400 (PEG) and water. The study was made based on the Extended Hildebrand Solubility Approach (EHSA) (5). Thus, this work is a continuation of previous research on acetaminophen in ethanol + water (6), propylene glycol + water (7), and ethanol + propylene glycol (8) mixtures. It is important to take into consideration that the EHSA method has been widely used to study the solubility of a lot of pharmaceutical compounds (9-27). On the other hand, PEG is after ethanol and propylene glycol the most used cosolvent to develop liquid pharmaceutical dosage forms (28). Moreover, PEG is also employed to regulate product evaporation (29).
THEORETICAL
The real solubility (X2) of a solid solute in a liquid solution is calculated adequately by means of the expression:
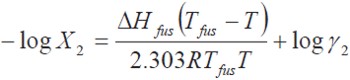 | [1] |
where, ΔHfus is the fusion enthalpy of the solute, R is the gas constant, Tfus is the melting point of the solute, T is the absolute temperature of the solution, log Υ2 is the non-ideality term. The Υ2 term is the activity coefficient of the solute and it is determined experimentally. One method of calculating γ2 is the referent to regular solutions obtained from
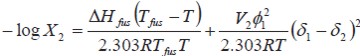 | [2] |
where V2 is the partial molar volume of the solute, ø1 is the volume fraction of the solvent in the saturated solution, and δ1 and δ2 are the solubility parameters of solvent and solute, respectively. Pharmaceutical dissolutions deviate from predicted by the regular solutions theory. In this respect, Martin et al. developed the EHSA method (9-15). If the A term (defined as V2ø/21(2.303RT ) is introduced in the Eq. [2], the real solubility of drugs can be calculated from the expression
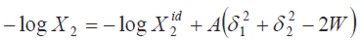 | [3] |
where the W term is equal to 2Kδ1δ2 (where, 2Kδ1δ2 is the Walker parameter). The W factor can be calculated from experimental data by means of
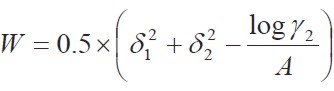 | [4] |
where Υ2 is the activity coefficient of the solute in the saturated solution, and it is calculated as Xid2/ X2 . The experimental values of the W parameter can be correlated by means of regression analysis by using regular polynomials as a function of δ1, as follows
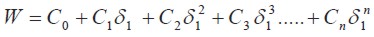 | [5] |
These empiric models can be used to estimate the drug solubility by means of back-calculation resolving this property from the specific W value obtained in the respective polynomial regression.
EXPERIMENTAL
Reagents and materials
Acetaminophen (Paracetamol, N-Acetyl- p-aminophenol, CAS RN: 103-90-2) was in agreement with the quality requirements of the American Pharmacopeia, USP (30). Polyethylene glycol 400 from DOW Chemicals (PEG), distilled water with conductivity < 2 mS cm–1, and filter units from Millipore Corp. Swinnex®-13 were also used.
Solvent mixtures preparation
The PEG employed was maintained over molecular sieve (Merck Number 3, 0.3 nm in pore diameter) to obtain a dry solvent prior to preparing the solvent mixtures. All PEG + water solvent mixtures were prepared in quantities of 50.00 g by mass using an Ohaus Pioneer TM PA214 analytical balance, in mass fractions from 0.10 to 0.90 varying by 0.10.
Solubility determination
An excess of ACP was added to each mixed solvent evaluated in stoppered dark glass flasks. Solid-liquid mixtures were placed on a thermostatic bath (Neslab RTE 10 Digital One Thermo Electron Company) kept at 298.15 K for at least 7 days to reach the saturation equilibrium. Once at equilibrium, supernatant solutions were filtered before analysis. ACP concentrations were determined by measuring UV-absorbance after appropriate gravimetric dilutions with water and interpolation from a previously constructed UV spectrophotometric calibration curve (UV/VIS BioMate 3 Thermo Electron Company spectrophotometer). Density of the saturated solutions was determined with a digital density meter (DMA 45 Anton Paar) according to the procedure described in the literature (31).
Estimation of the volumetric contributions
Apparent specific volumes ( φVspc ) of the drug were calculated according to Eq. [6], where, m2 and m1 are the masses of solute and solvent in the saturated solution, respectively, VE1 is the specific volume of the solvent, and ρsoln is the solution density (2).
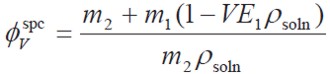 | [6] |
The ACP apparent molar volume is calculated by multiplying the øV φspc value and the molar mass of the solute.
RESULTS AND DISCUSSION
The information about polarity and volumetric behavior of PEG + water mixtures, as a function of the composition, is shown in Table 1. On the other hand, the reported ideal solubility for this drug is 2.602 ×10–2 in mole fraction (32). Table 1 also summarizes the ACP solubility expressed in molarity and mole fraction, the density of the solvent and saturated mixtures, the apparent molar volume of ACP, and the solvent volume fraction in the saturated solutions at 298.15 K. Figure 2 shows the experimental solubility and the calculated solubility by using the regular solution model as a function of the solubility parameter of solvent mixtures.
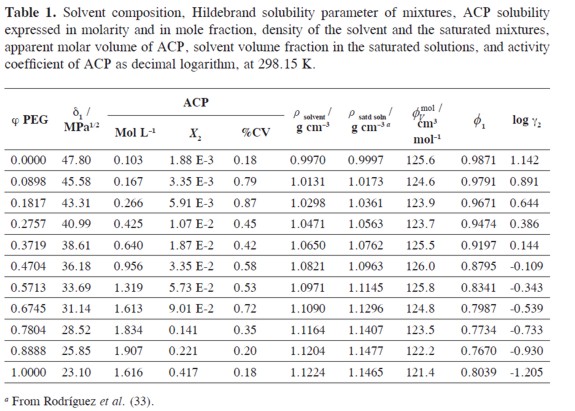
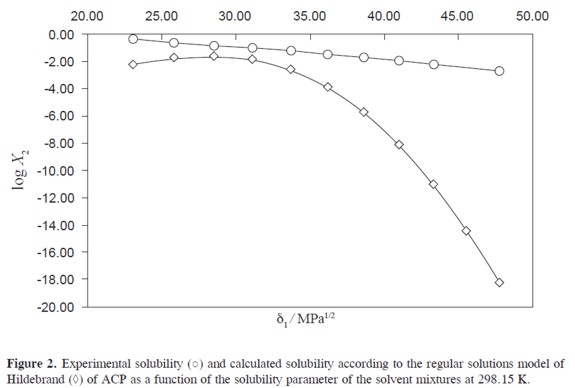
From density values of cosolvent mixtures and saturated solutions, in addition to ACP solubility, the solvent volume fraction (ø1) and apparent molar volume of the solute (øVmol) of the drug in the saturated mixtures, were calculated. These values are also presented in Table 1.
Ultimately, the activity coefficients of ACP as decimal logarithms are also presented in Table 1. These values were calculated from experimental solubility val ues and ideal solubility at 298.15 K (X2 = 2.602 × 10–2). In water rich mixtures, Υ2 values were greater than unit because the experimental solubilities are lower than the ideal value but in PEG rich mixtures these values were below one.
In order to calculate the W parameter, the solubility parameter of ACP (δ2) is required and for this reason it was calculated by using Fedors and Van Krevelen methods as showed in Table 2 (34) obtaining the value 27.3 MPa1/2 which is similar to that obtained experimentally in ethanol + water and ethanol (6) + propylene glycol mixtures (8), i.e. 28.0 MPa1/2. In the next calculations the experimental value was used. It is interesting that PEG, where the maximum drug solubility is obtained, has a lower δ value (23.1 MPa1/2) compared to ACP. This result demonstrates that the maximum solubility is not always obtained in mixtures where the solubility parameters of drug and solvent are coincident.
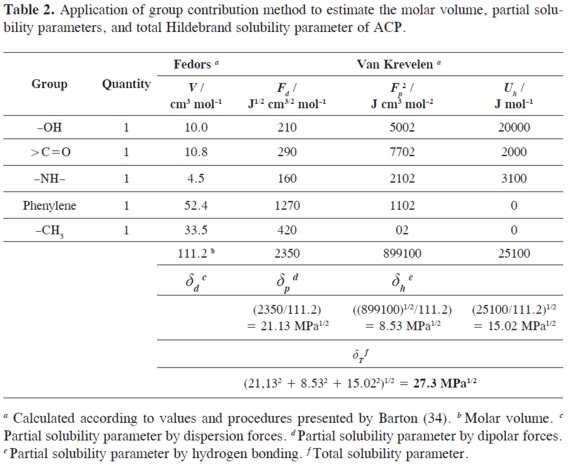
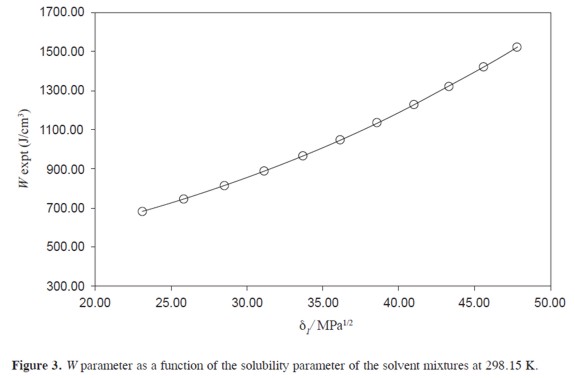
Table3 summarizes the parameters A, K, and W for ACP in PEG + water mixtures. Figure 3 shows that the variation of the W parameter with respect to the solubility parameter of solvent mixtures, presents deviation from linear behavior.

W values were adjusted to regular polynomials in orders from 1 to 5 (Eq.5). Table 4 summarizes the coefficients obtained in all the regular polynomials from degrees one to five, whereas the W values back-calculated by using the respective polynomials are presented in Table 5. It is clear that these values depend on the model used in the W back-calculation. Similar behaviors have been reported in the literature for this drug and for several other compounds in different solvent mixtures (6-27).
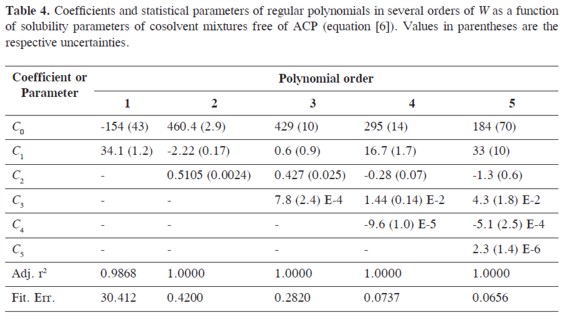
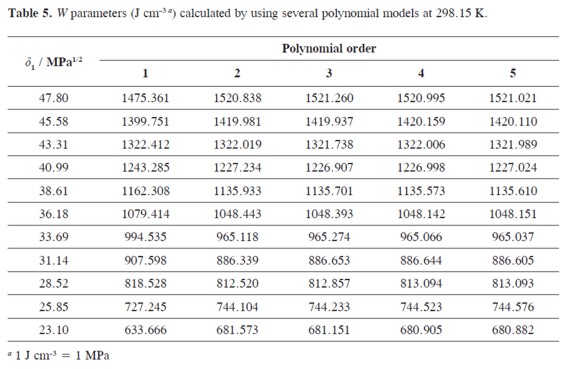
Table 6 summarizes the solubility values obtained by using the W values obtained by back-calculation from the polynomial models (Table 4) which are presented in Table 5. In the same way it was made previously (6-27) and because the best adjustment is being searched, the first criterion used to define the polynomial order of W term as function of δ1 was the fitting standard uncertainties obtained, which values were as follows, 30.4, 0.420, 0.282, 0.074, and 0.066 (Table 4), for orders one to five, respectively. As another comparison criterion, Table 6 also summarizes the percentages of difference between ACP experimental solubility and those calculated by using EHSA.
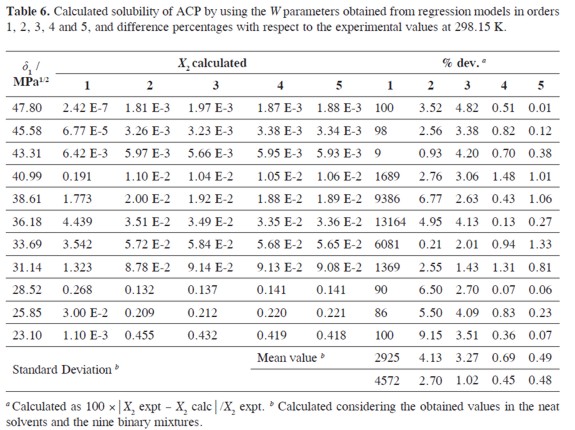
It was found that the more complex the polynomial used, the better the agreement found between experimental and calculated solubility. The most important increment in concordance is obtained when going from order 1 to order 2 (From 2925 to 4.13%). It is important to note that for pharmaceutical purposes an uncertainty below 5% is useful for practical purposes but for academic purposes a better agreement is required. In this way, the best improvement is obtained going from 3rd to 4th degree, i.e. from 3.27 to 0.69%. Thereby, in the following calculations the model in order 4 was used, just as has been made earlier on (26, 27). Nevertheless, it is interesting that the mean deviation using a polynomial of order 5 (0.49%, Table 6) is almost the same obtained as mean in the experimental uncertainties obtained (0.50%, Table 1)
As it has been described previously,an important consideration about the usefulness of the EHSA method is that which refers to justifying the complex calculations involving any other variables, instead of the simple empiric regression of the experimental solubility as a function of the solvent mixtures´ solubility parameters (Table 1, Figure 4). For this reason, in the Table 7 the experimental solubilities are confronted to those calculated directly by using a regular polynomial in order 4 of log X2 as a function of δ1 values (Equation [7],with adjusted determination coefficient r2 = 0.9998 and fitting standard uncertainty = 0.0111) and also to those calculated involving the W parameters obtained from Eq. [5] adjusted to order 4 (Tables 4 and 5). The respective difference percentages are also presented in Table 7.
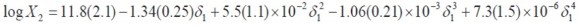 | [7] |
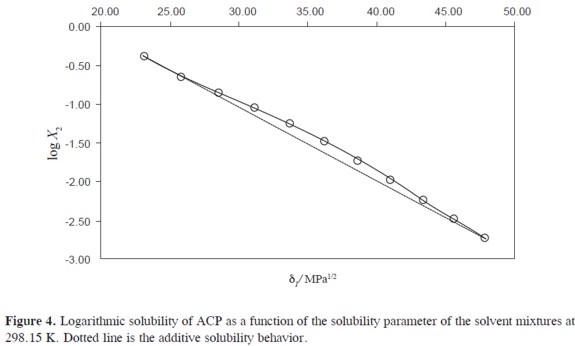
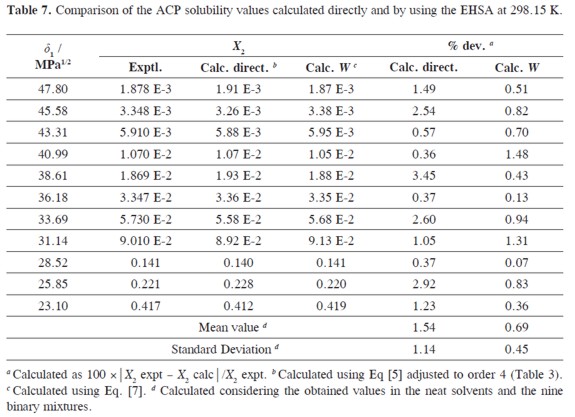
Based on mean deviation percentages presented in Table 6(1.54% and 0.69% for direct calculation and EHSA method, respectively) it follows that a slight difference is found between the values obtained by using both methods. As it has happened for several drugs, the present the EHSA method for practical and academic purposes, in particular, if differences below 1% are required.
On the other hand, it is very interesting that this drug mainly exhibits posi tive deviations with respect to the ideal log-linear additive model proposed by Yalkowsky and Roseman (dotted line in Figure 4) (3). This behavior is different compared to those observed by Rubino and Obeng (35) who found negative deviations in water-rich mixtures and positive deviations in propylene glycol-rich mixtures by studying the solubility of homologous series of some alkyl p-hydroxybenzoates and p-aminobenzoates. It is also different compared to those reported for ibuprofen, naproxen, ketoprofen, and indomethacin in the similar cosolvent mixtures (36-40) where negative and positive deviations were also found in water-rich and cosolvent-rich mixtures, respectively. The results for ACP in PEG mixtures could be attributed to a better solvation of the drug by the cosolvent molecules by means of hydrogen bonding where the phenolic hydroxyl group of ACP would be interacting with the ether groups of PEG.
CONCLUSION
The EHSA method has been adequately used in the present work to study the solubility of acetaminophen in PEG + water mixtures by using experimental values of molar volume and Hildebrand solubility parameter of this analgesic drug. In particular, a good predictive character has been found by using a regular polynomial in order four of the interaction parameter W as a function of the solubility parameter of solvent mixtures free of solute. In this way, the predictive character of EHSA is better than that obtained by direct correlation between solubility and mixtures composition.
ACKNOWLEDGEMENTS
The authors wish to thank the Department of Pharmacy of the Universidad Nacional de Colombia for facilitating the use of equipment and facilities used in this research work.
1. Raffa, R. B. Analgesic, antipyretic, and anti-inflammatory drugs. In: Remington: The Science and Practice of Pharmacy. 21 ed. A. Gennaro (ed). Philadelphia: Lippincott Williams & Wilkins. 2005. pp. 1524- 1542. [ Links ]
2. Jiménez, J. A.; Martínez, F. Thermodynamic study of the solubility of acetaminophen in propylene glycol + water cosolvent mixtures. J. Braz. Chem. Soc. 2006. 17: 125- 134. [ Links ]
3. Yalkowsky, S. H.; Roseman, T. J. Solubilization of drugs by cosolvents. In: Techniques of Solubilization of Drugs. S. H. Yalkowsky (ed). New York: Marcel Dekker, Inc. 1981. pp. 91-134. [ Links ]
4. Jouyban, A. Handbook of Solubility Data for Pharmaceuticals. Boca Raton, FL: CRC Press, Taylor & Francis Group. 2010. pp. 30-58. [ Links ]
5. Martin, A.; Bustamante, P.; Chun, A. H. C. Physical Chemical Principles in the Pharmaceutical Sciences. 4 ed. Philadelphia: Lea & Febiger,1993. [ Links ]
6. Romero, S.; Reillo, A.; Escalera, B.; Bustamante, P. The behavior of paracetamol in mixtures of amphiprotic and amphiprotic-aprotic solvents: Relationship of solubility curves to specific and nonspecific interactions. Chem. Pharm. Bull. 1996. 44: 1061-1064. [ Links ]
7. Martínez, F. Utilidad del método extendido de Hildebrand en el estudio de la solubilidad del acetaminofén en mezclas agua-propilenoglicol. Rev. Acad. Colomb. Cienc. 2005. 29: 429-438. [ Links ]
8. Martínez, F. Aplicación del método extendido de Hildebrand al estudio de la solubilidad del acetaminofén en mezclas etanol-propilenoglicol. Acta Farm. Bonaerense 2005. 24: 215-224. [ Links ]
9. Martin, A.; Newburger, J.; Adjei, A. Extended Hildebrand approach: Solubility of caffeine in dioxane-water mixtures. J. Pharm. Sci. 1980. 69: 659-661. [ Links ]
10. Martin, A.; Carstensen, J. Extended solubility approach: Solubility parameters for crystalline solid compounds. J. Pharm. Sci. 1981. 70: 170-172. [ Links ]
11. Martin, A.; Paruta, A. N.; Adjei, A. Extended Hildebrand Solubility Approach: Methylxanthines in mixed solvents. J. Pharm. Sci. 1981. 70: 1115-1115. [ Links ]
12. Martin, A.; Miralles, M. J. Extended Hildebrand solubility approach: Solubility of tolbutamide, acetohexamide, and sulfisomidine in binary solvent mixtures. J. Pharm. Sci. 1982. 71: 439-442. [ Links ]
13. Martin, A.; Wu, P. L.; Adjei, A.; Mehdizadeh, M.; James, K. C.; Metzler, C. Extended Hildebrand solubility approach: testosterone and testosterone propionate in binary solvents. J. Pharm. Sci. 1982. 71: 1334-1340. [ Links ]
14. Martin, A.; Wu, P. L. Extended Hildebrand solubility approach: p-Hydroxybenzoic acid in mixtures of dioxane and water. J. Pharm. Sci. 1983. 72: 587-592. [ Links ]
15. Martin, A.; Wu, P. L.; Velasquez, T. Extended Hildebrand solubility approach: sulfonamides in binary and ternary solvents. J. Pharm. Sci. 1985. 74: 277-282. [ Links ]
16. Jouyban-Gharamaleki, A.; Acree Jr., W. E. Comment concerning: solubility prediction of caffeine in aqueous N,N-dimethylformamide mixtures using the extended Hildebrand solubility approach. Int. J. Pharm. 1999. 177: 127-128. [ Links ]
17. Pacheco, D. P.; Manrique, Y. J.; Vargas, E. F.; Barbosa, H. J.; Martínez, F. Validez del método extendido de Hildebrand en la predicción de las solubilidades de ibuprofén y naproxén en mezclas propilenoglicol- etanol. Rev. Colomb. Quím. 2007. 36: 55-72. [ Links ]
18. Aragón, D. M.; Pacheco, D. P.; Ruidiaz, M. A.; Sosnik, A. D.; Martínez, F. Método extendido de Hildebrand en la predicción de l solubilidad de naproxeno en mezclas cosolventes etanol + agua. Vitae, Rev. Fac. Quím. Farm. 2008. 15: 113-122. [ Links ]
19. Ruidiaz, M. A.; Martínez, F. Método extendido de Hildebrand en la estimación de la solubilidad de la indometacina en mezclas acetato de etilo + etanol. Rev. Colomb. Quím. 2009. 38: 235-247. [ Links ]
20. Rathi, P. B. Prediction of satranidazole solubility in water-polyethylene glycol 400 mixtures using extended Hildebrand solubility approach. Iranian J. Pharm. Sci. 2010. 7, 17-24. [ Links ]
21. Gantiva, M.; Martínez, F. Método extendido de Hildebrand en la predicción de la solubilidad del ketoprofeno en mezclas cosolventes etanol + agua. Quím. Nova 2010. 33: 370-376. [ Links ]
22. Rodríguez, S. J.; Cristancho, D. M.; Neita, P. C.; Vargas, E. F.; Martínez, F. Extended Hildebrand solubility approach in the solubility estimation of the sunscreen ethylhexyl triazone in ethyl acetate + ethanol mixtures. Lat. Am. J. Pharm. 2010.29: 1113-1119. [ Links ]
23. Ruidiaz, M. A.; Delgado, D. R.; Mora, C. P.; Yurquina, A.; Martínez, F. Estimation of the indomethacin solubility in ethanol + water mixtures by the extended Hildebrand solubility approach. Rev. Colomb. Cienc. Quím. Farm. 2010. 39: 79-95. [ Links ]
24. Rathi, P. B.; Mourya, V. K. Extended Hildebrand solubility approach: Satranidazole in mixtures of dioxane and water. Indian J. Pharm. Sci. 2011. 73: 315-319. [ Links ]
25. Rathi, P. B. Prediction of satranidazole solubility in water-polyethylene glycol 400 mixtures using Extended Hildebrand Solubility Approach. Iranian J. Pharm. Sci. 2011. 7, 17- 24. [ Links ]
26. Ruidiaz, M. A.; Delgado, D. R.; Martínez, F. Extended Hildebrand solubility approach to correlate the indomethacin solubility in 1,4-dioxane + water mixtures. Quím. Nova 2011. 34: 1569-1574. [ Links ]
27. Holguín, A. R.; Delgado, D. R.; Martínez, F. Indomethacin solubility in propylene glycol + water mixtures according to the extended Hildebrand solubility approach. Lat. Am. J. Pharm. 2012. 31: 720-726. [ Links ]
28. Rubino, J. T. Cosolvents and cosolvency. In: Encyclopedia of Pharmaceutical Technology. Vol 3. J. Swarbrick; J.C. Boylan (eds). New York: Marcel Dekker, Inc. 1988. pp. 375-398. [ Links ]
29. Aulton, M. E. Pharmaceutics, The Science of Dosage Forms Design. 2 ed. London: Churchill Livingstone. 2002. [ Links ]
30. US Pharmacopeia. 23 ed. Rockville, MD: United States Pharmacopeial Convention, 1994. [ Links ]
31. Rodríguez, S. J.; Cristancho, D. M.; Neita, P. C.; Vargas, E. F.; Martínez, F. Volumetric properties of the octyl methoxycinnamate + ethyl acetate solvent system at several temperatures. Phys. Chem. Liq. 2010. 48: 638-647. [ Links ]
32. Baena, Y.; Pinzón, J. A.; Barbosa, H.; Martínez, F. Temperature dependence of the solubility of some acetanilide derivatives in several organic and aqueous solvents. Phys. Chem. Liq. 2004. 42: 603-613. [ Links ]
33. Rodríguez, G. A.; Holguín, A. R.; Martínez, F.; Khoubnasabjafari, M.; Jouyban, A. Volumetric properties of (PEG 400 + water) and (PEG 400 + ethanol) mixtures at several temperatures and correlation with the Jouyban-Acree model. Lat. Am. J. Pharm. 2012. Submitted. [ Links ]
34. Barton, A. Handbook of Solubility Parameters and Other Cohesion Parameters. 2nd ed. New York: CRC Press, 1991, pp. 157-193. [ Links ]
35. Rubino, J. T.; Obeng, E. K. Influence of solute structure on deviations from the log-linear solubility equation in propylene glycol water mixtures. J. Pharm. Sci. 1991. 80: 479-483. [ Links ]
36. Vargas, E. F.; Manrique, Y. J.; Pacheco, D. P.; Torres, N. S.; Martínez, F. Desviaciones al modelo logarítmico-lineal en la solubilidad de ibuprofén y naproxén en mezclas cosolventes propilenoglicol-agua. Quím. Nova 2007. 30: 1945-1950. [ Links ]
37. Vargas, E.; Sosnik, A.; Martínez, F. Aplicación del modelo de Jouyban Acree para la estimación de la solubilidad del naproxeno en mezclas cosolventes etanol + agua. Lat. Am. J. Pharm. 2008. 27: 654-660. [ Links ]
38. Gantiva, M.; Vargas, E. F.; Manzur, M. E.; Yurquina, A.; Martínez, F. Modelos de Yalkowsky Roseman y Jouyban Acree en la estimación de la solubilidad del ketoprofeno en algunas mezclas cosolventes propi lenoglicol + agua. Rev. Colomb. Cienc. Quím. Farm. 2009. 38: 156 171. [ Links ]
39. Gantiva, M., Yurquina, A., Martínez, F. Desempeño de los modelos de Yalkowsky & Roseman y de Jouyban & Acree en la estimación de la solubilidad del ketoprofeno en mezclas cosolventes etanol + agua. Vitae, Rev. Fac. Quím. Farm. 2009. 16: 361-368. [ Links ]
40. Ruidiaz, M. A.; Delgado, D. R.; Martínez, F. Performance of the Jouyban Acree and Yalkowsky Roseman models for estimating the solubility of indomethacin in ethanol + water mixtures. Rev. Acad. Colomb. Cienc. 2011. 35: 329-336. [ Links ]













