Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.42 no.1 Bogotá Jan./Apr. 2013
Preferential solvation of xylitol in ethanol + water co-solvent mixtures according to the IKBI and QLQC methods
Solvatación preferencial del xilitol en mezclas cosolventes etanol + agua según los métodos IKBI y QLQC
Solvatação preferencial do xilitol no misturas cosolventes etanol + água acordo o métodos IKBI e QLQC
Daniel R. Delgado1, Edgar F. Vargas2, Fleming Martínez1*
1 Grupo de Investigaciones Farmacéutico Fisicoquímicas, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, A.A. 14490, Bogotá D.C., Colombia.
2 Grupo de Termodinámica de Soluciones, Departamento de Química, Facultad de Ciencias, Universidad de los Andes, Bogotá D.C., Colombia.
* Autor para correspondencia: fmartinezr@unal.edu.co
Abstract
The preferential solvation parameters, i.e., the differences between the local around the solute and bulk mole fractions of the solvents in solutions of xylitol in ethanol + water binary mixtures are derived from their thermodynamic properties by means of the inverse Kirkwood-Buff integrals (IKBI) and quasi-lattice quasi-chemical (QLQC) methods. According to IKBI method it is found that xylitol is sensitive to solvation effects, so the preferential solvation parameter δxE,S, is slightly positive in water-rich and negative in mixtures beyond 0.25 in mole fraction of ethanol. In different way, according to QLQC method, negative values of δxE,S are found in all the compositions evaluated. The more solvation by ethanol observed in water-rich mixtures could be due mainly to polarity effects. Otherwise, the preference of this compound for water in ethanol-rich mixtures could be explained in terms of the bigger acidic behavior of water interacting with hydrogen-acceptor hydroxyl groups in xylitol.
Keywords: Xylitol, ethanol, solubility, IKBI, QLQC, preferential solvation.
Resumen
Partiendo de algunas propiedades termodinámicas clásicas se calcularon los parámetros de solvatación preferencial del xilitol (δxE,S) en mezclas etanol + agua mediante el método de las integrales inversas de Kirkwood-Buff (IKBI) y el método cuasi-enrejado-cuasi-químico (QLQC). Los parámetros δxE,S corresponden a las diferencias entre las fracciones molares locales alrededor del soluto y en el grueso de la solución. Con base en estos valores, se encuentra que este compuesto es altamente sensible a los efectos específicos de solvatación según la composición cosolvente. Así, según el método IKBI, los valores de δxE,S son positivos en mezclas ricas en agua pero negativos en composiciones desde 0.25 en fracción molar de etanol hasta el etanol puro. Sin embargo, según el método QLQC, los valores de δxE,S son negativos en todas las composiciones co-solventes analizadas. En mezclas ricas en agua la mayor solvatación por las moléculas de etanol podría deberse principalmente a efectos de polaridad. De otro lado, la preferencia que manifiesta este compuesto por el agua en mezclas ricas en etanol podría explicarse en términos del mayor comportamiento ácido del agua que estaría interactuando con los grupos aceptores de hidrógeno presentes en el soluto.
Palabras clave: xilitol, etanol, solubilidad, IKBI, QLQC, solvatación preferencial.
Resumo
Começando a partir de algumas propriedades termodinâmicas clássicos neste trabalho, foram calculados os parâmetros de solvatação preferenciais de xilitol (δxE,S) em misturas etanol + água pelo método de integrais inversas de Kirkwood-Buff (IKBI) e o método quase-reticulado quase-químicas (QLQC). Parâmetros δxE,S correspondem às diferenças entre as fracções molares locais ao redor do soluto na solução e a granel. Com base nestes valores se verifique que este composto é extremamente sensível aos efeitos específicos de solvatação por composição de cosolvente. Assim, de acordo com o método IKBI, valores δx E,S são positivas em misturas ricas em água, mas negativas em composições 0,25 de fracção mole de etanol a etanol puro. No entanto, de acordo com o método QLQC, os valores de δxE,S são negativas em todas as composições testadas. Solvatação de xilitol por moléculas de etanol em misturas ricas em água pode ser devido por polaridade. Por outro lado, a preferência de este composto por água em misturas em etanol pode ser explicada em termos de comportamento ácido de água com os grupos aceitadores de hidrogénio presentes no soluto.
Palavras-chave: xilitol, etanol, a solubilidade, IKBI, QLQC, solvatação preferencial.
Introducción
Xylitol (2R,4S)-Pentane-1,2,3,4,5-pentol, CAS RN 87-99-0) is an alditol commonly used as additive in several cosmetic and nutraceutical formulations and products (Figure 1). As nutrient agent it is given as oral and intravenous way (1). This compound has been used as a sweetener for diabetic patients and it is actively beneficial for dental health by reducing caries to a third when used regularly. Thus, this sweetening agent is finding increasing application in chewing gum, mouth-rinses, and toothpastes. Unlike sucrose, xylitol is not fermented into cariogenic acid end products (2). According to the US Pharmacopeia it is classified as an official pharmaceutical aid (3).
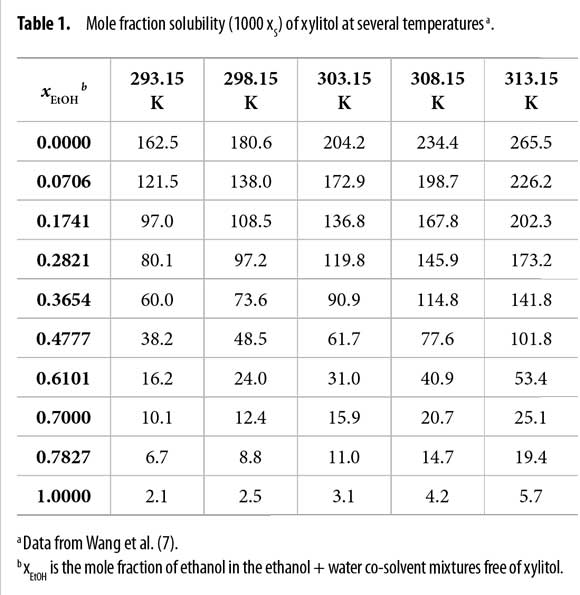
Normally xylitol is obtained from xylose by following several biotechnological procedures (4-6). In its industrial manufacture, xylitol is purified through crystallization from solution as the final step and dilution crystallization generally is preferable. Aqueous alcoholic mixtures are widely used for this purpose. Because the knowledge about solubility is crucial for crystallization processes, Wang et al. (7) studied the xylitol solubility in several ethanol + water mixtures at several temperatures.
Solubility of drugs and other pharmaceutical ingredients, as well as cosmetically and food ingredients, in co-solvent mixtures knowledge is very important for scientists involved in several development stages such as drug and excipients purification and design of liquid medicines (8). Although co-solvency has been employed in pharmacy for several decades it is recently that the mechanisms involved to increase or decrease organic compounds solubility have been approached from a physicochemical point of view (9). In this way, a recent thermodynamic work has been published based on the enthalpic and entropic contributions to the Gibbs energy of solution of this sweetener agent (7). Nevertheless, the preferential solvation, i.e., the co-solvent specific composition around the xylitol molecules has not been studied. Therefore, the main goal of this paper is to evaluate the preferential solvation of xylitol in ethanol + water co-solvent mixtures, based on some classical thermodynamic definitions. Thus, this work is similar to the ones presented previously in the literature for some analgesic drugs in similar co-solvent mixtures (10-13).
The inverse Kirkwood-Buff integral (IKBI) is a powerful tool for evaluating the preferential solvation of nonelectrolytes in solvent mixtures, describing the local compositions around a solute with respect to the different components present in the solvent mixture (14-16). In similar way, quasi-lattice quasi-chemical (QLQC) approach is also useful to do this although is not too much exact as IKBI is (17).
The first treatment depends on the values of the standard molar Gibbs energies of transfer of the solute xylitol from neat water to the ethanol + water co-solvent mixtures and the excess molar Gibbs energy of mixing for the co-solvent binary mixtures. As has been indicated previously, this treatment is very important in pharmaceutical sciences to understand the molecular interactions solute-solvent because most of the solubility studies developed have been directed towards correlating or modeling the solubilities, and possibly predicting them, from the solubilities in the neat solvents, but not to analyze the local environment around the solute molecules describing the local fraction of the solvent components (E or W) in the surrounding of solute (S) (18, 19).
In the second case, the QLQC method, proposed by Marcus (17), supposes that the number of nearest neighbors a molecule has (the lattice parameter Z) is the weighted mean of the lattice parameter of the pure components. It also presumes that the interaction energy of a molecule of any component with others is independent of the nature of the other neighbors. The model also assumes that ideal volumes and entropies of mixing take place (VExc = 0; SExc = 0). The main advantage of this method is that non-derivative functions are required as in the case of IKBI method (17).
In this work the IKBI and QLQC approaches are applied to evaluate the preferential solvation of xylitol in the binary mixtures conformed by ethanol (E or EtOH) and water (W). The results are expressed in terms of the preferential solvation parameter δx E,S of the solute by the two solvent components.
Theoretical
The KBIs (Kirkwood-Buff integrals, Gi,S) are given by the following expression:
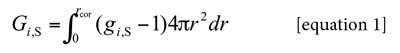
Here g i,S is the pair correlation function for the molecules of the solvent i in the ethanol + water mixtures around the solute xylitol, r the distance between the centers of the molecules of xylitol and ethanol or water, and rcor is a correlation distance for which gi,S (r > rcor) ≈ 1. Thus, for all distances r > rcor up to infinite, the value of the integral is essentially zero. Therefore, the results are expressed in terms of the preferential solvation parameter δxi,S for the solute in solution by the component solvents ethanol and water (20). For ethanol (E) this parameter is defined as:
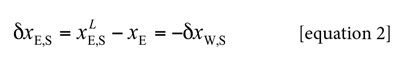
Where xE is the mole fraction of ethanol in the bulk solvent mixture and x LE,S is the local mole fraction of ethanol in the environment near to the solute. If δxE,S > 0 then the solute xylitol is preferentially solvated by ethanol; on the contrary, if it is < 0 the solute is preferentially solvated by water, within the correlation volume, Vcor =(4π/3)r3cor, and the bulk mole fraction of ethanol, xE. Values of δxE,S are obtainable from those of GE,S, and these in turn, from thermodynamic data of the co-solvent mixtures with the solute dissolved on it, as shown below (18).
Algebraic manipulation of the basic expressions presented by Newman (20) leads to expressions for the Kirkwood-Buff integrals (in cm3 mol-1) for the individual solvent components in terms of some thermodynamic quantities as shown in equations [3] and [4] (15, 18, 19):
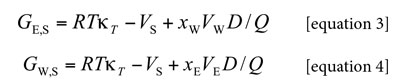
Where κT is the isothermal compressibility of the ethanol + water solvent mixtures (in GPa-1), VE and VW are the partial molar volumes of the solvents in the mixtures (in cm3 mol-1), similarly, VS is the partial molar volume of xylitol in these mixtures (in cm3 mol-1). The function D is the derivative of the standard molar Gibbs energies of transfer of the solute (from neat water to ethanol + water mixtures) with respect to the solvent composition (in kJ mol-1, as also is RT) and the function Q involves the second derivative of the excess molar Gibbs energy of mixing of the two solvents (GExc E+W) with respect to the water proportion in the mixtures (also in kJ mol-1) (18-21):
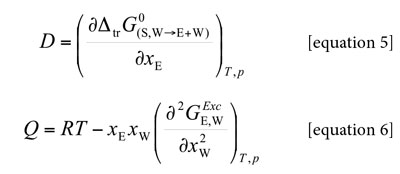
Because the dependence of kT on composition is not known for a lot of the systems investigated and because of the small contribution of RT kT to the IKBI the dependence of kT on composition could be approximated by considering additive behavior according to the equation [7] (22):
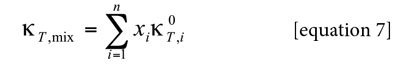
Where xi is the mole fraction of component i in the mixture and k0T,i is the isothermal compressibility of the pure component i.
Ben-Naim (15) showed that the preferential solvation parameter could be calculated from the Kirkwood-Buff integrals as follows:
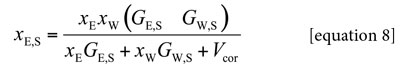
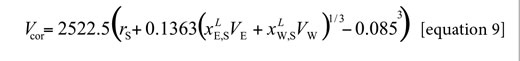
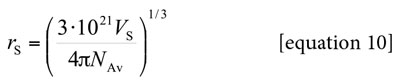
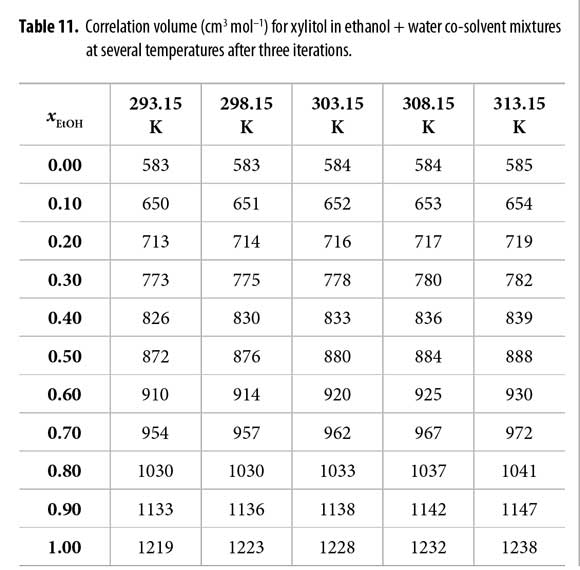
Here, NAv is the Avogadro number. However, the definitive correlation volume requires iteration, because it depends on the local mole fractions. This iteration is done by replacing δxE,S in the equation [2] to calculate X L E,S until a non-variant value of Vcor is obtained.
For the QLQC method, the local mole fraction of solvent component ethanol around the xylitol molecules is defined as (17, 19):
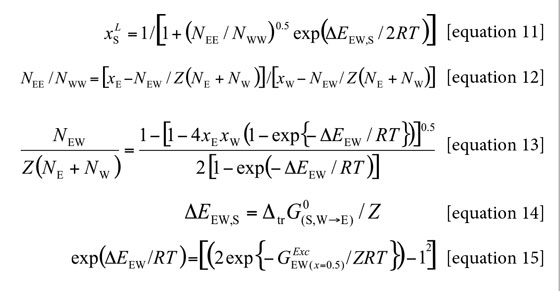
In these equations, the lattice parameter Z is usually assumed as 10. NE and NW are the number of molecules of both components in the bulk, whereas, NEE, NWW, and NEW are the number of neighboring pairs of these molecules in the quasi lattice. Equation [14] expresses the difference in the molar neighbor interaction energies of xylitol with the solvents ethanol and water, ΔEEW,S, by the molar Gibbs energy of transfer from water to ethanol per neighboring lattice. ΔEEW denotes the molar energy of interaction of solvent on neighboring quasi-lattice sites. It is important to note that only the Gibbs energy of the xylitol transfer between the neat solvents and the excess Gibbs energy of mixing at equimolar composition of both solvents are required for this method.
Results and discussion
The solubility of xylitol in ethanol + water mixtures (Table 1) was taken from Wang et al. (7). Xylitol solubility diminishes with the increasing the proportion of ethanol in the mixtures at all the temperatures studied. It is fo r this reason that this compound is purified by precipitation after adding ethanol to its aqueous solutions.
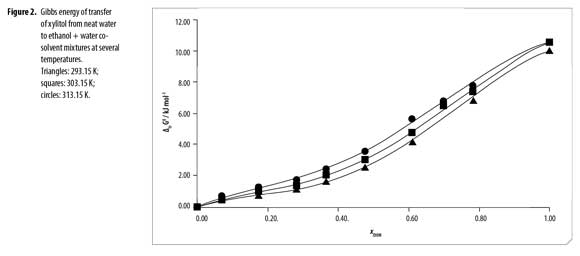
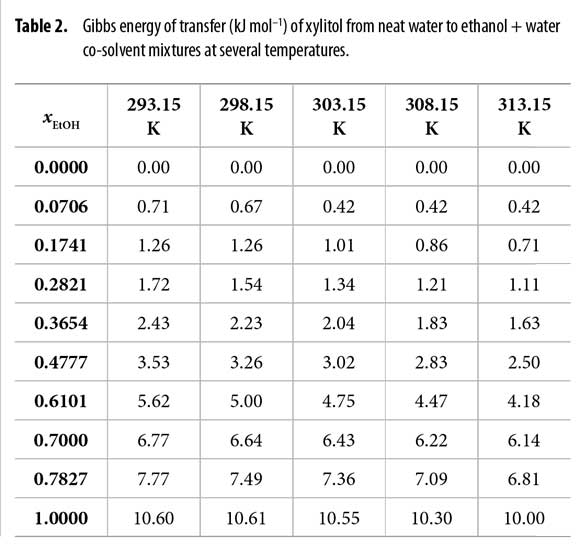
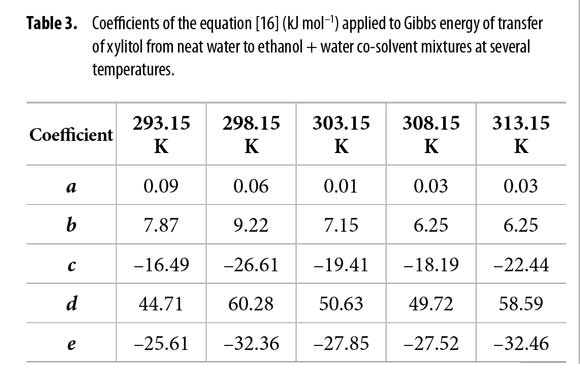
Standard molar Gibbs energy of transfer of this sweetening agent from neat water to ethanol + water mixtures is calculated and correlated to regular quartic polynomials from the drug solubility data by using equation [16]. Figure 2 shows the Gibbs energy of transfer behavior at several temperatures whereas Table 2 shows the behavior at all the temperatures studied. Otherwise, polynomials coefficients are shown in Table 3.
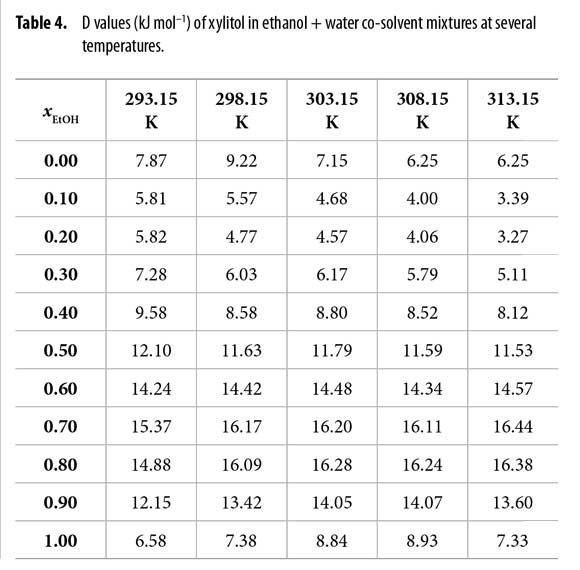
Thus D values are calculated from the first derivative of the polynomial models (equation [17]) solved according to the co-solvent mixtures composition. This procedure was done varying by 0.05 in mole fraction of ethanol but in the following tables the respective values are reported varying only by 0.10 in mole fraction. D values are reported in Table 4.
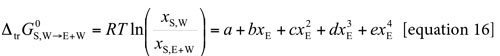
In order to calculate the Q values the excess molar Gibbs energies of mixing GExc E,W at all the temperatures considered are required. Nevertheless, normally these values are reported only at one temperature, i.e. 298.15 K. For this reason, it is necessary to calculate it at other temperatures. In this way, GExc E,W values were calculated at 298.15 K by using the equation [18] as reported by Marcus (18). On the other hand, the GExc E,W values at the other temperatures were calculated by using the equation [19], where, HExcE,W is the excess molar enthalpy of the co-solvent mixtures, T1 is 298.15 K and T2 is one of the other temperatures under consideration (18). In turn, HExcE,W values were calculated by using the equation [20] at 298.15 K as also reported by Marcus (18).
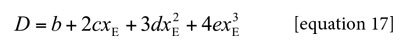
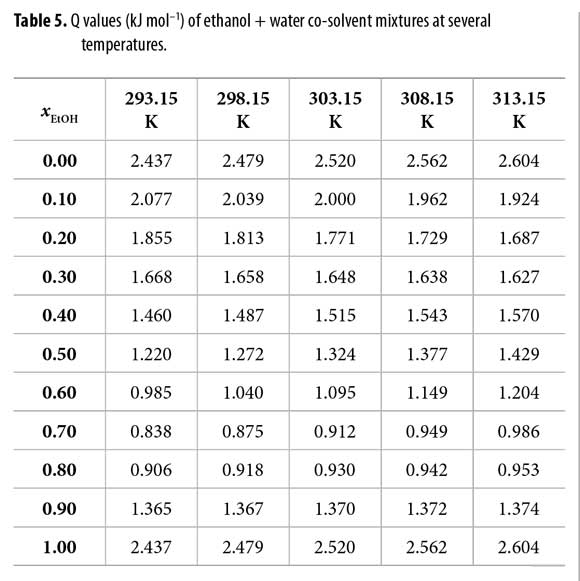
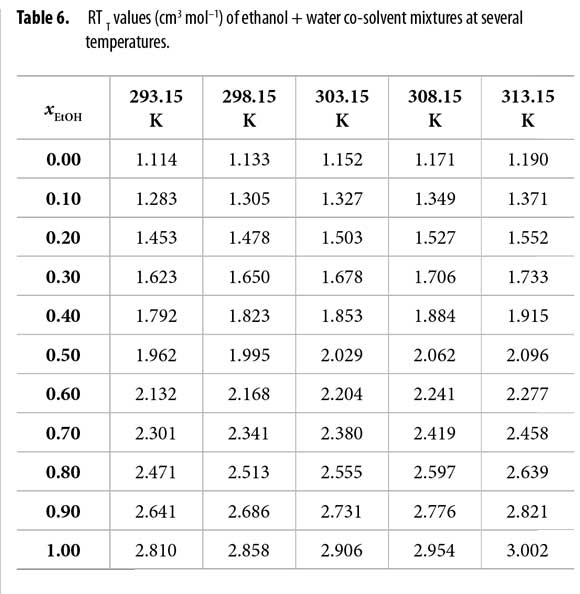
It is important to note that quartic regular polynomials of GExcE,W as a function of the mole fraction of water were obtained. Q values at all temperatures are shown in Table 5. On the other hand, Table 6 shows the RT kT values calculated by assuming additive behavior of kT (equation [7]) with the values 1.153 and 0.457 GPa-1, for ethanol and water, respectively (22).
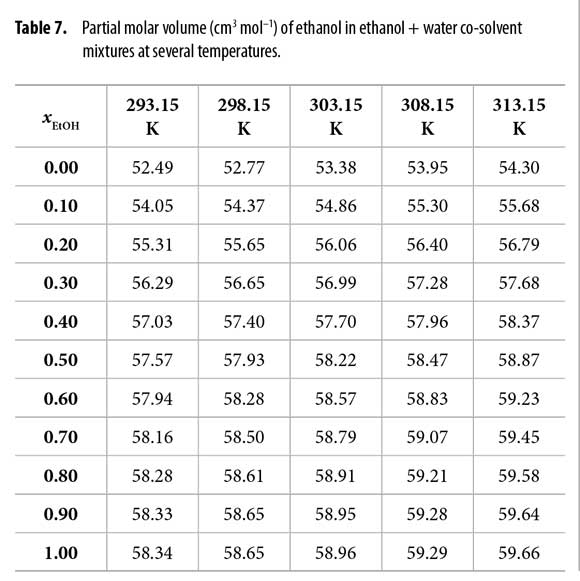
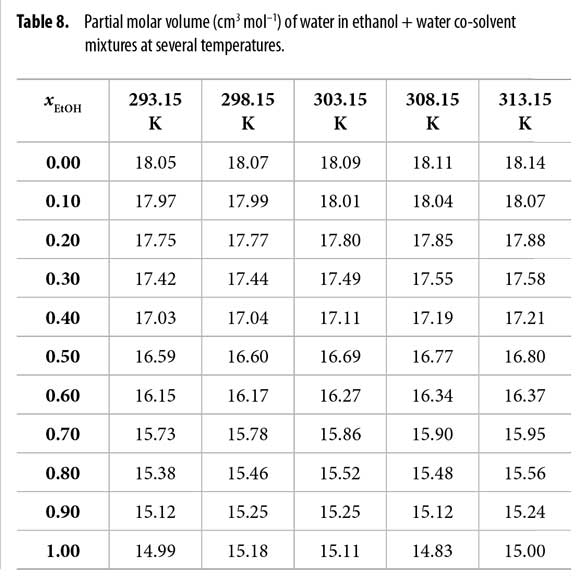
The partial molar volumes of ethanol (Table 7) and water (Table 8) were calculated by means of equations [21] and [22] from the density (ρ) values of ethanol + water mixtures reported by Jiménez et al. at all the temperatures under study (23). In these equations V is the molar volume of the mixtures and it is calculated as V = (xE ME + xW·MW)/ρ. ME and MW are 46.06 and 18.02 g mol-1, respectively (1).
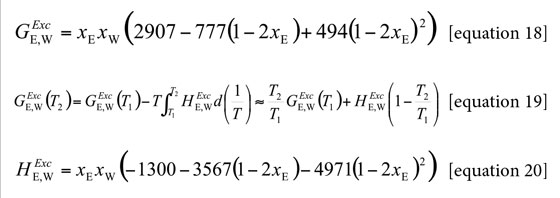
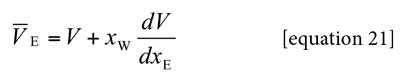
Partial molar volumes of non-electrolyte drugs are not frequently reported in the literature. This is because this property is dependent on temperature and composition and therefore it requires a lot of experiments. For this reason, in a first approach the molar volume of xylitol is considered here as independent of co-solvent composition and temperature, and equal to that presented in solid state, just as it is calculated by considering the density value reported in the literature (1.52 g cm-3) (2) obtaining the value 100.10 cm3 mol-1. Additionally, from this volume value the radius of the drug molecule (required for equation [9]) was calculated by using the equation [10] as rS = 0.341 nm.
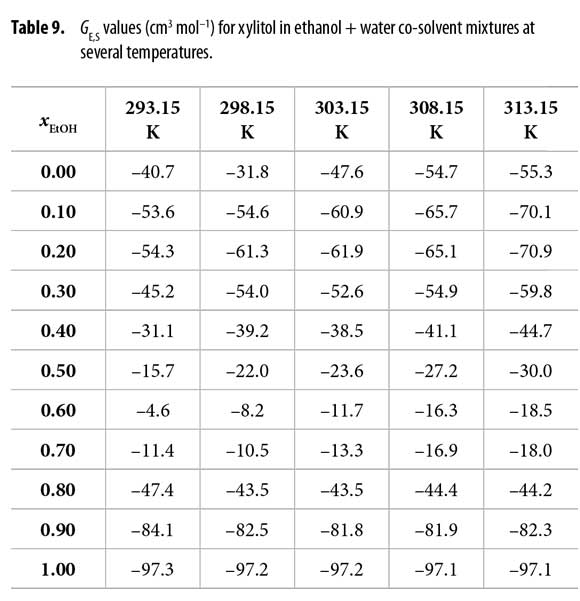
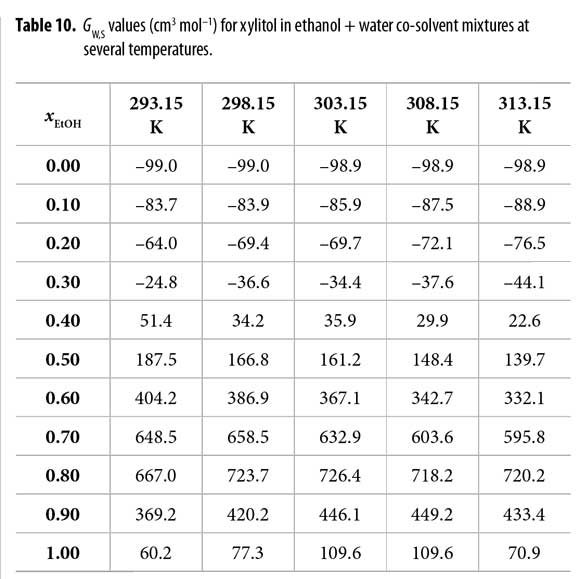
Table 9 shows that all the GE,S values are negative with the maximum in xEtOH = 0.60. In different way, Table 10 shows that GW,S values are negative in water-rich mixtures but positive beyond the mixture with xEtOH = 0.40 reaching maximum value in the mixture with xEtOH = 0.80. This could be interpreted as the preference of xylitol by water in ethanol-rich mixtures.
In order to use the IKBI method, the correlation volume was iterated three times by using the equations [2], [8] and [9] to obtain the values reported in Table 11. It is interesting to note that this value is almost independent on temperature in water-rich mixtures but increases in some extent in ethanol-rich mixtures. This could be a consequence of the greater molar expansibility of ethanol in comparison with water (23).
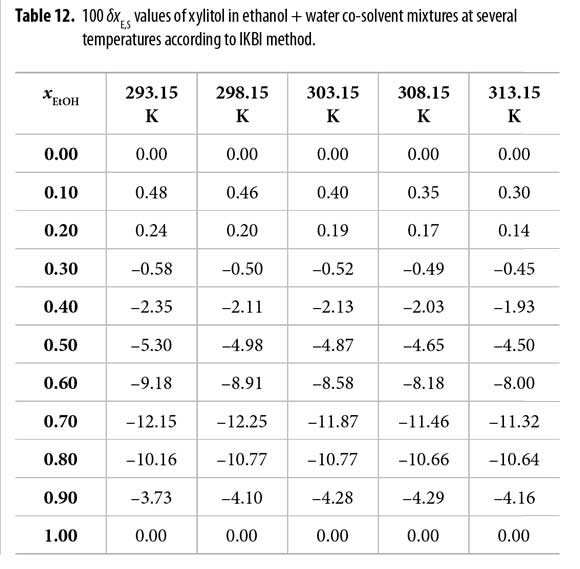
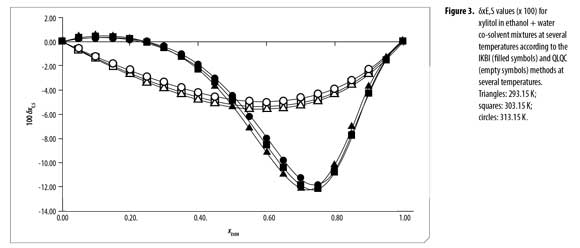
The values of δxE,S vary non-linearly with the ethanol concentration in the aqueous mixtures at all the temperatures studied (Table 12 and Figure 3). In the beginning, the addition of ethanol to water tends to make positive the δxE,S values of xylitol from the pure water up to the mixture 0.25 in mole fraction of ethanol reaching a maximum of 4.8 x 10 -3. This maximum diminishes with the temperature increasing.
From this ethanol proportion up to neat ethanol, the local mole fraction of the ethanol around the solute decreases, being the δxE,S values negative, and therefore, xylitol is preferentially solvated by water.
Xylitol acts in solution as a Lewis acid due to the hydrogen atoms in its -OH groups (see Figure 1) in order to establish hydrogen bonds with proton-acceptor functional groups in the solvents (oxygen atoms in -OH). In addition, this drug could act as a Lewis base due to free electron pairs in oxygen atoms of hydroxyl groups to interact with acidic hydrogen atoms in both solvents.
According to the preferential solvation results, it is conjecturable that in water-rich mixtures, the xylitol is acting as Lewis acid with ethanol molecules because this co-solvent is more basic than water, i.e. the Kamlet-Taft hydrogen bond acceptor parameters are β = 0.75 for ethanol and 0.47 for water (24). On the other hand, in ethanol-rich mixtures, where the solute is preferentially solvated by water, this compound is acting mainly as a Lewis base in front to water because the Kamlet- Taft hydrogen bond donor parameters are, α = 1.17 for water and 0.86 for ethanol, respectively (25). Thus, water is more acidic than ethanol. In this way, the specific and nonspecific interactions between xylitol and the co-solvent decrease in these mixtures (12, 26).
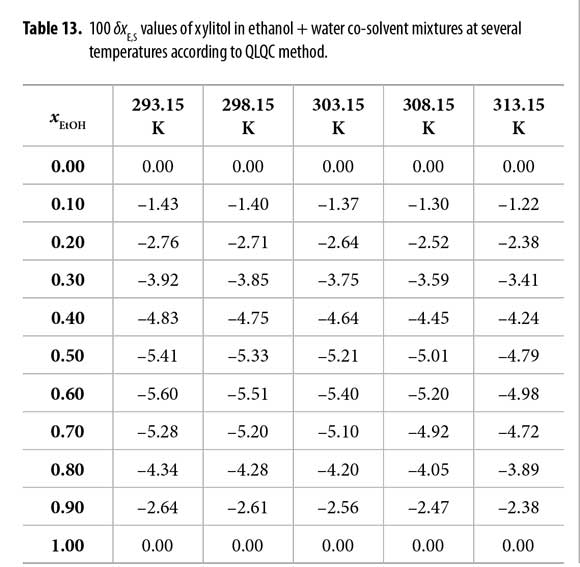
On the other hand, in order to use the QLQC method, the excess Gibbs energy of mixing of equimolar mixture of both solvents was used as follows: 0.709, 0.727, 0.744, 0.762, and 0.780 kJ mol-1, at temperatures from 293.15 to 313.15 K, respectively (12). According to QLQC method (Table 13 and Figure 3) xylitol is preferentially solvated by water in all the mixtures and the δxE,S values are bigger (as negative magnitude) than the ones obtained by using the IKBI method in mixtures with composition 0.25 < xEtOH < 0.50 but they are lower in the other mixtures (0.50 < xEtOH < 1.00). Therefore, as has been indicated in the literature the IKBI method is more indicate than QLQC to discriminate the effect of co-solvent composition on the local mole fraction around the drugs molecules (12, 13). Nevertheless, it is important to keep in mind that QLQC requires only two specific values, i.e. Gibbs energy of transfer of xylitol from water to ethanol and the excess Gibbs energy of mixing at xEtOH = 0.50, so it is more easy to use.
Conclusions
Explicit expressions for local mole faction mof ethanol and water around of xylitol were derived on the basis of the IKBI and QLQC methods applied to equilibrium solubility values of this sweetening agent in ethanol + water mixtures. Thus, this compound is preferentially solvated by ethanol in water-rich mixtures but preferentially solvated by water in mixtures beyond 0.25 in mole fraction of ethanol at all temperatures considered. These results are in agreement with that described previously and base in more classical thermodynamic treatments (7).
References
1. Budavari, S.; O'Neil, M.J.; Smith, A.; Heckelman, P.E.; Obenchain Jr., J.R.; Gallipeau, J.A.R.; D'Arecea, M.A. The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th edition. Whitehouse Station, NJ: Merck & Co., Inc., 2001. [ Links ]
2. Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients. 5th edition London: Pharmaceutical Press, 2006. [ Links ]
3. US Pharmacopeia. 23 ed. Rockville, MD: United States Pharmacopeial Convention, 1994. [ Links ]
4. Converti, A.; Perego, P.; Dominguez, J.M. Xylitol production from hardwood hemicellulose hydrolysates by Pachysolen tannophilus, Debaryomyces hansenii, and Candida guilliermondii. Appl. Biochem. Biotechnol. 1999. 82: 141-151. [ Links ]
5. Sreenivas Rao, R.; Pavana Jyothi, Ch.; Prakasham, R.S.; Sarma, P.N.; Venkateswar Rao, L. Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresource Technol. 2006. 97: 1974-1978. [ Links ]
6. Rambo, M.K.D.; Bevilaqua, D.B.; Brenner, C.G.B.; Martins, A.F.; Mario, D.N.; Alves, S.H.; Mallmann, C.A. Xylitol from rice husks by acid hydrolysis and Candida yeast fermentation. Quim. Nova 2013. 36: 634-639. [ Links ]
7. Wang, Zh.; Wang, Q.; Liu, X.; Fang, W.; Li, Y.; Xiao, H. Measurement and correlation of solubility of xylitol in binary water+ethanol solvent mixtures between 278.00 K and 323.00 K. Korean J. Chem. Eng. 2013. 30: 931-936. [ Links ]
8. Jouyban, A. Handbook of Solubility Data for Pharmaceuticals. Boca Raton, FL: CRC Press, 2010. [ Links ]
9. Rubino, J.T. Cosolvents and cosolvency. In: Encyclopedia of Pharmaceutical Technology. Vol 3. Swarbrick, J.; Boylan, J.C. (eds). New York: Marcel Dekker, Inc. 1988. pp. 375-398. [ Links ]
10. Marcus, Y. Preferential solvation of ibuprofen and naproxen in aqueous 1,2-propanediol. Acta Chim. Slov. 2009. 56. 40-44. [ Links ]
11. Ruidiaz, M.A.; Delgado, D.R.; Martínez, F.; Marcus, Y. Solubility and preferential solvation of indomethacin in 1,4-dioxane + water solvent mixtures. Fluid Phase Equilib. 2010. 299: 259-265. [ Links ]
12. Delgado, D.R.; Holguín, A.R.; Almanza, O.A.; Martínez, F.; Marcus, Y. Solubility and preferential solvation of meloxicam in ethanol + water mixtures. Fluid Phase Equilib. 2011. 305: 88-95. [ Links ]
13. Holguín, A.R.; Delgado, D.R.; Martínez, F.; Marcus, Y. Solution thermodynamics and preferential solvation of meloxicam in propylene glycol + water mixtures. J. Solution Chem. 2011. 40: 1987-1999. [ Links ]
14. Ben-Naim, A. Theory of preferential solvation of nonelectrolytes. Cell Biophysics 1988. 12: 255-269. [ Links ]
15. Ben-Naim, A. Preferential solvation in two- and in three-component systems. Pure Appl. Chem. 1990. 62: 25-34. [ Links ]
16. Marcus, Y. Solubility and solvation in mixed solvent systems. Pure Appl. Chem. 1990. 62: 2069-2076. [ Links ]
17. Marcus, Y. Preferential solvation in mixed solvents. In: Fluctuation Theory of Solutions: Applications in Chemistry, Chemical Engineering, and Biophysics. Smith, P.E.; Matteoli, E.; O'Connell, J.P. (eds). Boca Raton: CRC Press, Taylor & Francis Group, 2013. [ Links ]
18. Marcus, Y. Solvent Mixtures: Properties and Selective Solvation. New York: Marcel Dekker, Inc., 2002. [ Links ]
19. Marcus, Y. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 2008. 140: 61-67. [ Links ]
20. Newman, K.E. Kirkwood-Buff solution theory: Derivation and applications. Chem. Soc. Rev. 1994. 23: 31-40. [ Links ]
21. Cortez-Nunez, N.G. Caracterização das Interacções Soluto (Ou Substrato)-Solvente-Solvente em Misturas Ternárias. Ph.D. thesis, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, Lisboa, 2010. [ Links ]
22. Marcus, Y. The Properties of Solvents. Chichester: John Wiley & Sons, 1998. [ Links ]
23. Jiménez, J.; Manrique, J.; Martínez, F. Effect of temperature on some volumetric properties for ethanol + water mixtures. Rev. Colomb. Cienc. Quím. Farm. 2004. 33: 145-155. [ Links ]
24. Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The beta-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976. 98: 377-383. [ Links ]
25. Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. II. The alpha-scale of solvent hydrogen-bond donor (HBA) acidities. J. Am. Chem. Soc. 1976. 98, 2886-2894. [ Links ]
26. Ruckenstein, E.; Shulgin, I. Effect of a third component on the interactions in a binary mixture determined from the fluctuation theory of solutions. Fluid Phase Equilib. 2001. 180: 281-297. [ Links ]