Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.43 no.3 Bogotá Sept./Dec. 2014
Human intestinal mucus proteins isolated by transanal irrigation and proctosigmoidoscopy.
Proteínas del moco intestinal humano aislado por irrigación transanal y rectosigmoidoscopia
Proteinas do muco intestinal humano isolado por irrigação transanal e retossigmoidoscopia
Gómez-Buitrago Paola-Andrea PhD*1, González-Correa Carlos-Augusto PhD1, Santacoloma-Osorio Mario MD2, Taborda-Ocampo Gonzalo PhD1 y Zezzi-Arruda Marco-Aurelio PhD3.
1 Research Group on Electrical Bio-Impedance, Doctoral Program on Biomedical Sciences, University of Caldas, Calle 65 # 26 – 10, Edificio de Laboratorios, Office 502, Manizales, Colombia, South America.
2 Clinical Department, University of Caldas, Carrera 25 # 48-57, Manizales, Colombia, South America.
3 State University of Campinas, (UNICAMP), Institute of Chemistry, Campinas, Brazil. Telefax +57-6-(8781500), extention 14160
* Autor de correspondencia: quimicapao@gmail.com
Article citation:
Gómez-Buitrago, P. A.; González-Correa, C. A.; Santocoloma-Osorio, M.; Taborda-Ocampo, G.; Zezzi-Arruda, M. A. Human intestinal mucus proteins isolated by transanal irrigation and proctosigmoidoscopy. Rev Colomb Quim. 2014. 43(3): 5-12.
Recibido: 20 de septiembre de 2014. Aceptado: 25 de octubre de 2014
Abstract
Human intestinal mucus essentially consists of a network of Mucin2 glycoproteins embedded in many lower molecular weight proteins. This paper contributes to the proteomic study of human intestinal mucus by comparing two sample collection methods (transanal irrigation and brush cytology during proctosigmoidoscopy) and analysis techniques (electrophoresis and digestion in solution). The entire sample collection and treatment process is explained, including protein extraction, digestion and desalination and peptide characterisation using a nanoAcquity UPLC chromatograph coupled to an HDMS spectrometer equipped with a nanoESI source. Collecting mucus via transanal irrigation provided a larger sample volume and protein concentration from a single patient. The proctosigmoidoscopy sample could be analysed via digestion in solution after depleting albumin. The analysis indicates that a simple mucus lysis method can evaluate the electrophoresis and digestion in solution techniques. Studying human intestinal mucus complexes is important because they perform two essential survival functions for humans as the first biochemical and physical defences for the gastrointestinal tract and a habitat for intestinal microbiota, which are primarily hosted in the colon and exceeds the human genetic information and cell number 100- and 10-fold (1).
Keywords: digestion in solution, electrophoresis, intestinal mucus, proctosigmoidoscopy, proteins, transanal irrigation.
Resumen
El moco intestinal humano está formado, esencialmente, por una red de la glicoproteína Mucina2, en la cual se encuentran inmersas muchas otras proteínas de menor peso molecular. El objetivo principal del presente trabajo es realizar una contribución al estudio proteómico del moco intestinal humano, mediante la comparación de dos métodos de recolección de muestra (irrigación transanal y cepillos de citología durante rectosigmoidoscopia) y de dos técnicas de análisis (electroforesis y digestión en solución). Se explica todo el proceso de recolección y tratamiento de la muestra para extraer las proteínas, la digestión y desalinización de las mismas, hasta llegar a la caracterización de los péptidos en un cromatógrafo nanoAcquity UPLC acoplado a un espectrómetro HDMS, equipado con una fuente nanoESI. La recolección del moco por irrigación transanal aportó mayor volumen de muestra de un solo paciente y mayor concentración de proteínas. La muestra obtenida por rectosigmoidoscopia se pudo analizar por digestión en solución, previa depleción de albúmina. El análisis indica que una metodología sencilla de lisis del moco puede ser utilizada para evaluación por las técnicas de electroforesis y de digestión en solución. El estudio del complejo moco intestinal humano es importante por las dos funciones primordiales que cumple para la supervivencia del ser humano: como la primera línea de defensa bioquímica y física del tracto gastrointestinal, y como el hábitat de la microbiota intestinal, la cual se hospeda principalmente en el colon, y supera en información genética hasta 100x la del humano y en 10x su número de células (1).
Palabras clave: digestión en solución, electroforesis, irrigación transanal, moco intestinal, proteínas, recto-sigmoidoscopia.
Resumo
O muco intestinal humano está formado essencialmente por uma rede da glicoproteína Mucin2, constituída por outras proteínas de menor massa molecular. O objetivo principal deste trabalho é contribuir ao estudo proteômico do muco mediante a comparação de dois métodos de amostragem (irrigação transanal e escovas de citologia durante retossigmoidoscopia) e dois técnicas de análise (e digestão em solução). São descritos os processos de amostragem, extração, digestão e dessalinização de proteínas até chegar na caracterização dos peptídeos digeridos em um cromatógrafo nanoAcquity UPLC acoplado a um espectrómetro HDMS, equipado com uma fonte nanoESI. A coleta do muco por irrigação transanal forneceu um volume maior de amostra por pessoa e maior concentração de proteínas. As amostras obtidas por retossigmoidoscopia foram analisadas por digestão em solução, após a depleção de albumina. As análises mostraram que uma metodologia simples de lise do muco pode ser utilizada para avaliação mediante as técnicas eletroforese e digestão em solução. O estudo do complexo MIH é importante pelas suas duas funções fundamentais na sobrevivência humana, sendo a primeira linha de defesa bioquimica e física do trato gastrointestinal, e o habitat da microbiota intestinal, hospedada principalmente no cólon e que contem informação genética até 100 X superior à do humano e 10 X seu número de células (1).
Palabras clave: digestão em solução, eletroforese, irrigação transanal, muco intestinal, proteínas, retossisgmoidoscopia.
Introduction
Proteomic studies conducted so far using different body fluids such as blood, (2), semen (3), cerebrospinal fluid, (4), saliva (5), and cervical mucus (6) have proven to significantly impact biomarker discovery and the clinical diagnosis and treatment of certain diseases. Human intestinal mucus (HIM) is a biologically important matrix defined as a gel formed from a complex mixture of water, glycoproteins, proteins, electrolytes, fatty acids and cells. Its basic structure consists of Muc2 mucin, which bonds glycans to synthesise a glycoprotein-type macromolecule resistant to endogenous proteases and digestive enzymes. Muc2 in human, mouse and rat colon mucus contain approximately 5200 amino acids and, when fully O-glycosylated in the serine and threonine domains that form the protein core interspersed with proline, can reach a mass of up to 2.5 MDa; therefore, Muc2 contains up to 80% glycans (7). Biosynthesis begins with Muc2 dimerising in the endoplasmic reticulum before the serines and threonines O-glycosylate in the Golgi apparatus and ending with trimer formation via the N-terminal, which yields a net-shaped structure where small proteins are immersed among other substances (8),(9). Mucus in the small intestine is more permeable to bacteria but has a higher concentration of antibacterial peptides and proteins secreted from Paneth cells and enterocytes such as lysozyme, α-defensins, histatins and lectins. Mucus in the colon acts as a physical and biochemical barrier to bacteria, food debris, by-products from bacteria and other hosts, enzymes and many other substances. In this organ, the mucus consists of two layers, a completely sterile inner layer facing the epithelium that is impervious to bacteria and an outer layer facing the lumen that is a habitat for bacteria. The inner and outer layers in the colon share Muc2 as their main mucin (7). In the inner layer, the glycans that join Muc2 are the first barrier to organisms (10),(11) because of the small pore sizes from the high Muc2 concentration; therefore, bacterial entry is not allowed. The outer layer is neither compact nor structured because it has a lower Muc2 concentration; therefore, bacteria can penetrate and attach to it. For this, they take advantage of its glycans, which act as adhesion substrates. These glycans provide not only a favourable microenvironment but also an energy source because bacteria use their enzymes to degrade polysaccharides and other glycoconjugates into oligosaccharides and short-chain fatty acids. Despite the current interest into this biofluid, other mucus components, including Clca3 and Agr2 proteins (essential disulphide isomerase for in vivo production of Muc2 (12)), Fcgbp, which is covalently bound to Muc2 and functions weave around other molecules, immunoglobulins, and many other proteins (13), were discovered and studied in 2008. In this study, we compared two methods to obtain and treat HIM, including its isolation and cleaning, protein extraction, SDS-PAGE separation and protein characterisation via digestion in solution, to assist studying this complex matrix.
Materials And Methods
Protocol of patient care
HIM was collected from six (6) patients admitted to a care program for overweight people that included a colon cleanse and proctosigmoidoscopy. Cleansing took five days during which the patients performed a supplemented fast; ingested bentonite (montmorillonite), psyllium and probiotics; and 1 (one) daily transanal irrigation. Patients signed their informed consent as approved by the Bioethics Committee of the Faculty of Health Sciences at the University of Caldas where the treatments were administered under medical supervision.
Daily transanal irrigation
During the five (5) days of colon cleansing, daily transanal irrigation was administered by volunteers by introducing body-temperature water via the rectum (14), (15) using a kit from the Colema Boards® Company (California, USA) following the manufacturer’s instructions (http://www.colema.com).
Collection and processing of the sample
The presence of intestinal mucus in the eliminated material from the patients during transanal irrigation was observed on days 3, 4 and 5 of the treatment. The sample was carefully isolated from the rest of the material using tweezers and suctioned using a Pasteur pipette. The mucus was cleaned by mixing with a cleaning buffer (16) in a 1:1 proportion before centrifuging at 350 g for 5 minutes and discarding 50 µL of supernatant. The centrifugation was repeated until a clear supernatant was obtained. The bacterial cleaning of the mucus was verified using a Gram stain for the remnant and supernatant. The clean mucus was stored at -80°C for later analysis. On the third day, each patient underwent a proctosigmoidoscopy after transanal irrigation to collect mucus from the colon. The doctor took the sample directly from the rectosigmoid wall via brush cytology. These samples underwent the same cleaning process as those obtained by transanal irrigation.
Protein extraction
Proteins were extracted from HIM using a denaturing buffer containing 8 M urea, 2% (w/v) CHAPS, 1% (w/v) DTT, 50 mM Tris pH 7.0 and 15 µL of PMFS per 300 µL of sample. Lysis was performed for 10 minutes.
SDS-PAGE
The proteins were cleaned by precipitation using the ReadyPrep 2-D Clean-up of Bio-Rad™ cleaning kit following the manufacturer’s instructions. The obtained precipitate was cleaned with 100 µL of cold 90% acetone by vortexing for one minute and stored for 10 minutes at -20°C; the cleaning process was performed three (3) times. The samples were centrifuged at 350 g for 5 minutes, and the obtained precipitate was dissolved in 100 µL of rehydration buffer containing 7 M urea, 2 M thiourea, 2% (w/v) CHAPS, 50 mM DTT and 1% (w/v) ampholyte (17). The proteins were quantified (18). The protein fraction was evaluated via a one-dimensional electrophoresis on 7-cm gels containing 5 µL of sample and 5 µL of a loading buffer containing 0.5 M Tris-HCl, 10% (m/v) glycerol, 10% (v/v) SDS, 0.001% (m/v) bromophenol blue and 5% (v/v) ß-mercaptoethanol. The run used a Mini-PROTEAN® 3 Cell (Bio Rad) electrophoresis system at 6.5 mA/gel under a constant 150-200 volts for 1 hour. Finally, the gels were stained with Coomassie colloidal solution and scanned using a Pharos FX Molecular Imager (Bio-Rad).
Albumin depletion and digestion in solution
A total of 150 µL of 53% (v/v) ACN were added to 100 µL of the protein solution extracted from the HIM using the denaturing buffer without cleaning as for SDS-PAGE (19). This simple was vortexed for one minute and left in an ultrasound bath for 10 minutes for 2 (two) repeats. Finally, the solution was centrifuged at 12000 g for 10 minutes, the precipitate was discarded, and the supernatant underwent the entire digestion in solution process (20).
Desalination of the obtained peptides via digestion in solution
The peptides were desalinated using C18 Allcrom columns with 100 mg/mL of reference 8B-S001-EAK and a manifold. The columns were activated using 3 mL of ACN at 100% and equilibrated with 1 mL of 50/50 v/v ACN/H2O (0.1% v/v formic acid) before adding 3 mL of 0.1% (v/v) TFA. The sample was subsequently loaded, and the column was washed with 3 mL of 0.1% (v/v) TFA and equilibrated with 1 mL of 0.1% formic acid (v/v). The elution used 2 mL of 50/50 v/v ACN/H2O (0.1% v/v formic acid). The eluent was collected again after adding 1 mL of 80/20 v/v ACN/H2O (0.1% v/v formic acid). The flow rate was 1 drop/s based on the Protocol of the National Laboratory of Biosciences of Brazil (21).
Mass spectrometry to identify proteins
Thesamples were concentrated to approximately 50 μL using a Speed Vac Concentrator model SPD131DDA (Thermo Scientific). The peptides in the digested sample were analysed on a UPLC nanoAcquity Waters chromatograph coupled to a Waters SYNAPT HDMS spectrometer equipped with a nanoESI source. A total of 2 to 5 μL of the aqueous sample was injected using the UPLC autosampler and placed in a guard pre-column (Waters Symmetry C18, 20 mm × 180 µm) for desalination with a 5 µL/min flow of 97/3 v/v H2O/ACN (0.1% v/v formic acid) for 3 minutes. The sample was then transferred to an analytical column (Waters BEH130 C18, 100 mm × 100 µm i.d., 1.7 µm particles) and eluted with a 1 µL/min flow. The peptides were detected in line using a mass spectrometer configured to operate in data dependent acquisition (DDA) mode using an MS function (fullscan of m/z 200 to 2000), three MS/MS functions, and an external calibration curve function (lockmass). The spectrometer operated under the following parameters: 3 kV capillary voltage, 30 V cone voltage, 100°C source temperature, 0.5 L/h nanoESI gas flow, 6 and 4 eV collision energy for the Trap and Transfer cells, respectively, and 1700 V detector. The MS (fullscan) and MS/MS (spectrum of ions fragmented by collision-induced dissociation) spectra were acquired at a rate of 1 spectrum/s. The instrument was calibrated before the analyses using a phosphoric acid oligomer (0.05% H3PO4 solution v/v in 50/50 v/v ACN/H2O) with an m/z from 90 to 1960. Argon at 9.7.10-3 mbar was used as the collision gas. The LC-MS runs were processed using ProteinLynx Global Server v.2.2 software (Waters), and all mass spectra were analysed using a peak list format in the MASCOT program v.2.2. (Matrix Science, London, UK). The selected search parameter was digestion ignoring trypsin until reaching a cleavage site. Methionine residue oxidation and peptides and fragment mass tolerance of ± 0.1 Da were used as modification variables. Searches used the SwissProt database.
Results and discussion
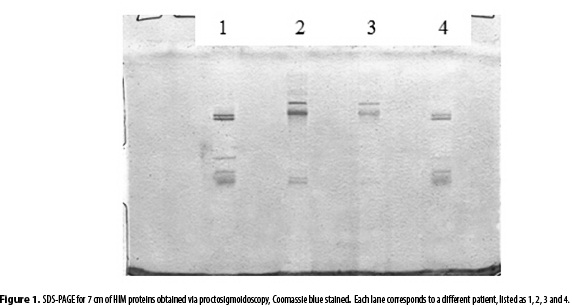
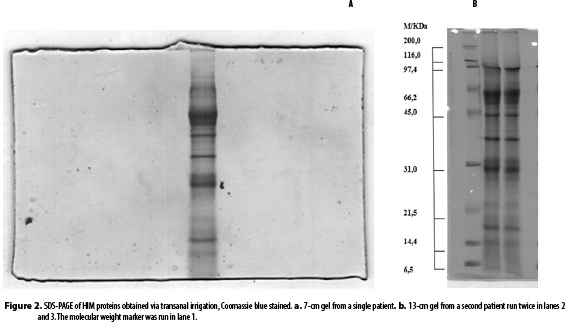
A proctosigmoidoscopy yields between 5 to 150 µL of mucus per patient, while a transanal irrigation session, yields from 0.5 mL to 5.0 mL of intestinal mucus. As shown in Figure 1, the proctosigmoidoscopy SDS-PAGE exhibited between 2 and 5 bands compared to the 13 bands for mucus obtained via transanal irrigation (figures 2a and 2b). The proctosigmoidoscopy samples exhibited a protein concentration of 1.059 µg/µL, 1.122 µg/µL, 0.197 µg/µL and 0.647 µg/µL, while the transanal irrigation sample exhibited a protein concentration of 22.4 µg/µL.
Figures 1 and 2 reveal a difference between the number of SDS-PAGE bands for the proctosigmoidoscopy and transanal irrigation samples. This effect was attributed to the different protein concentrations. The proctosigmoidoscopy sample has a low protein concentration, which prevents several bands from displaying after the staining, precipitation and dilution procedures. In contrast, the transanal irrigation yields a higher HIM volume, protein concentration and number of SDS-PAGE bands.
The digestion procedure was performed without depleting the albumin solution for two samples (one from transanal irrigation with a 22.4 µg/µL protein concentration, and another from the proctosigmoidoscopy with a 1.059 µg/µL protein concentration), which were characterised, and no protein was found in the proctosigmoidoscopy HIM. In contrast, the transanal irrigation sample for two patients yielded Galectin-4 for the sample of first patient and immunoglobulins IgA and IgJ for the sample of second patient.
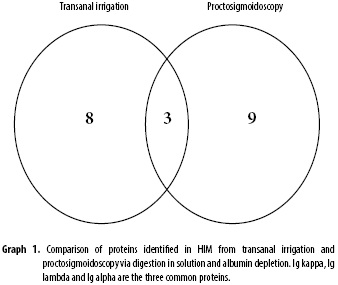
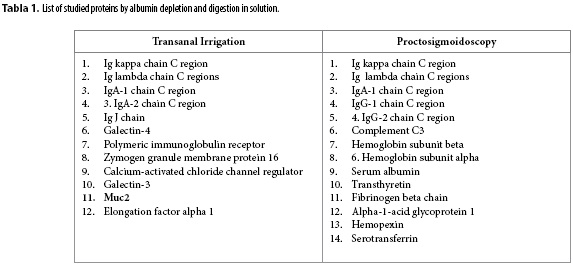
The entire digestion in solution process was performed on two samples (the same transanal irrigation sample and another proctosigmoidoscopy containing 1.122 µg/µL protein) including an albumin depletion and digestion, desalination and characterisation, which identified 20 (twenty) proteins (see Table 1 and supplementary materials 1 and 2), including Muc2, which is the main protein in intestinal mucus. This mucin, which belongs to the inner colonic mucus layer, is insoluble in the chaotropic salt GuHCl (13), (22) but is soluble in this salt when from the outer layer. Apparently, the proposed method, which includes a simple lysis buffer, partially denatures and reduces disulphide bonds, which can detect certain peptides via mass spectrometry.
Of the twenty identified proteins, five are of possible clinical interest: Complement C3 (23); Galectin-3 (24); Galectin-4 (25); Muc2 (26), (27) and Zymogen granule membrane protein 16 (28).
Conclusions
The colonic transanal irrigation procedure yields a sufficient quantity and quality of HIM samples from a single patient for SDS-PAGE, digestion in solution, and undoubtedly other analyses, such as 2D-PAGE, for differential studies. The proctosigmoidoscopy and transanal irrigation method for collecting HIM can be used for digestion in solution provided abundant proteins such as albumin are depleted.
This study presented two approaches for collecting, processing and analysing HIM. The first separates low molecular weight proteins because of the limited range for gels, and the second directly digests them in the extraction solution before sample desalinisation and digestion, which has the advantage of characterising high molecular weight proteins.
Characterising proteins obtained via digestion in solution can yield the "insoluble" Muc2, which was identified using ultracentrifugation and agarose gels (29), (13), (22).
References
1. Bäckhed F, Ley RE, Sonnenburg JL, Peterson D, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(2005):1915-20. [ Links ]
2. Bansal N, Gupta A, Sankhwar SN, Mahdi AA. Low- and high-grade bladder cancer appraisal via serum-based proteomics approach. Clin Chim Acta. Elsevier B.V.; 2014 Sep 25;436:97-103. [ Links ]
3. Sharma R, Agarwal A, Mohanty G, Jesudasan R, Gopalan B, Willard B, et al. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reprod Biol Endocrinol. Reproductive Biology and Endocrinology; 2013 Jan;11(1):38. [ Links ]
4. Chiasserini D, van Weering JRT, Piersma SR, Pham T V, Malekzadeh A, Teunissen CE, et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics. Elsevier B.V.; 2014 Jun 25;106:191-204. [ Links ]
5. Habte HH, Mall AS, de Beer C, Lotz ZE, Kahn D. The role of crude human saliva and purified salivary MUC5B and MUC7 mucins in the inhibition of Human Immunodeficiency Virus type 1 in an inhibition assay. Virol J. 2006 Jan;3:99. [ Links ]
6. Zegels G, Van Raemdonck G, Coen EP, Tjalma W, Van Ostade XWM. Comprehensive proteomic analysis of human cervical-vaginal fluid using colposcopy samples. Proteome Sci. 2009 Jan;7:17. [ Links ]
7. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008/09/23 ed. 2008;105(39):15064-9. [ Links ]
8. Factor PW, Gum JR, Hicks JW, Toribara NW, Siddiki B. Molecular Cloning of Human Intestinal Mucin ( MUCB ) cDNA. J Biol Chem. 1994;269(4):2440-6. [ Links ]
9. Asker N, Axelsson M, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998 Jul 24;273(30):18857-63. [ Links ]
10. Johansson ME V, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. Nature Publishing Group; 2013 Jun;10(6):352-61. [ Links ]
11. Ouwerkerk JP, de Vos WM, Belzer C. Glycobiome: bacteria and mucus at the epithelial interface. Best Pract Res Clin Gastroenterol. Elsevier Ltd; 2013 Feb;27(1):25-38. [ Links ]
12. Park S, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT. The protein disulfide isomerase AGR2 is essential. Proc Natl Acad Sci U S A. 2009;106(17):6950-5. [ Links ]
13. Johansson ME, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res. 2009/05/13 ed. 2009;8(7):3549-57. [ Links ]
14. Andrei Krassioukov, MD, PhD F, 1, 2, 3, 4 5, , Janice J. Eng, PhD Bs (PT/OT), 1, 3, 4, 5 6, Claxton R, 5, et al. Neurogenic bowel management after spinal cord injury: A systematic review of the evidence. Spinal Cord. 2011;48(10):718-33. [ Links ]
15. Funda DP. Management of neurogenyc bowel dysfunction. Eur Phys Rehabil Med. 2011;47(4):661-75. [ Links ]
16. Hamer HM, Jonkers DM, Loof A, Vanhoutvin SA, Troost FJ, Venema K, et al. Analyses of human colonic mucus obtained by an in vivo sampling technique. Dig Liver Dis. 2009/02/14 ed. 2009;41(8):559-64. [ Links ]
17. Panicker G, Ye Y, Wang D, Unger ER. Characterization of the Human Cervical Mucous Proteome. Clin Proteomics. 2010/05/13 ed. 2010;6(1-2):18-28. [ Links ]
18. Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54. [ Links ]
19. Kay R, Barton C. Enrichment of low molecular weight serum proteins using acetonitrile precipitation for mass spectrometry based proteomic analysis. Rapid Commum Mass Spectrom. 2008;3255-60. [ Links ]
20. Ribeiro D a, Cota J, Alvarez TM, Brüchli F, Bragato J, Pereira BMP, et al. The Penicillium echinulatum secretome on sugar cane bagasse. PLoS One. 2012 Jan;7(12):e50571. [ Links ]
21. Laboratorio de Espectrometría de Masas. Brasil. 2012. http://lnbio.wpengine.com/wp-content/uploads/2012/11/Protocolo-para-Dessaliniza%C3%A7%C3%A3o-de-Pept%C3%ADdeos.pdf. [ Links ]
22. Rodríguez-Piñeiro AM, Post S Van Der, Johansson ME V, Thomsson K a, Nesvizhskii AI, Hansson GC. Proteomic study of the mucin granulae in an intestinal goblet cell model. J Proteome Res. 2012 Mar 2;11(3):1879-90. [ Links ]
23. Dowling P, Clarke C, Hennessy K, Torralbo-Lopez B, Ballot J, Crown J, et al. Analysis of acute-phase proteins, AHSG, C3, CLI, HP and SAA, reveals distinctive expression patterns associated with breast, colorectal and lung cancer. Int J Cancer. 2012 Aug 15;131(4):911-23. [ Links ]
24. Kim D-W, Kim KH, Yoo BC, Hong S-H, Lim YC, Shin Y-K, et al. Identification of mitochondrial F(1)F(0)-ATP synthase interacting with galectin-3 in colon cancer cells. Cancer Sci. 2008 Oct;99(10):1884-91. [ Links ]
25. Barrow H, Rhodes JM, Yu L-G. Simultaneous determination of serum galectin-3 and -4 levels detects metastases in colorectal cancer patients. Cell Oncol (Dordr). 2013 Feb;36(1):9-13. [ Links ]
26. Van der Sluis M, De Koning B E, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006 Jul;131(1):117-29. [ Links ]
27. Fu J, Wei B, Wen T, Johansson ME V, Liu X, Bradford E, et al. Loss of intestinal core 1 - derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121(4):1657-66. [ Links ]
28. Tateno H, Yabe R, Sato T, Shibazaki A, Shikanai T, Gonoi T, et al. Human ZG16p recognizes pathogenic fungi through non-self polyvalent mannose in the digestive system. Glycobiology. 2012 Feb;22(2):210-20. [ Links ]
29. Johansson ME V, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011 Mar 15;108 Suppl :4659-65. [ Links ]