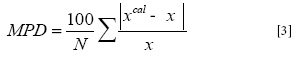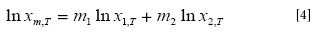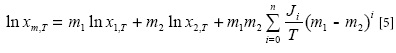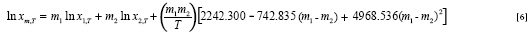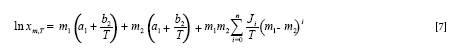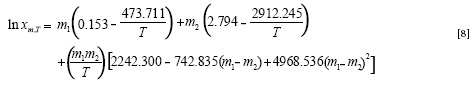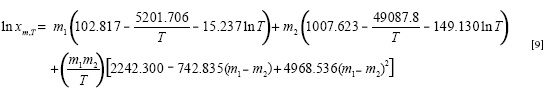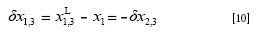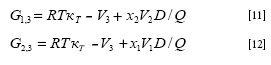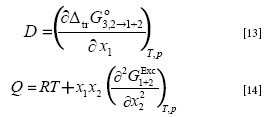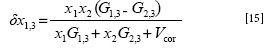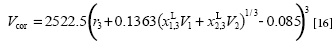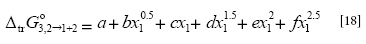Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.44 no.2 Bogotá May/Aug. 2015
https://doi.org/10.15446/rev.colomb.quim.v44n2.55218
DOI: http://dx.doi.org/10.15446/rev.colomb.quim.v44n2.55218
Some numerical analyses on the solubility of vanillin in Carbitol® + water solvent mixtures
Algunos análisis numéricos sobre la solubilidad de la vainillina en mezclas cosolventes Carbitol® + agua
Alguns análises numéricas da solubilidade de vanilina em misturas de solventes carbitol® + água
Fleming Martínez1,*, Abolghasem Jouyban2,3, William E. Acree Jr.4
1 Grupo de Investigaciones Farmacéutico-Fisicoquímicas, Departamento de Farmacia, Universidad Nacional de Colombia -Sede Bogotá, Cra. 30 No. 45-03, Bogotá D.C., Colombia.
2 Pharmaceutical Analysis Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz 51664, Iran
3 Kimia Idea Pardaz Azarbayjan (KIPA) Science Based Company, Tabriz University of Medical Sciences, Tabriz 51664, Iran
4 Department of Chemistry, University of North Texas, Denton, TX 76203-5070, USA
* Corresponding author: fmartinezr@unal.edu.co
Article citation:
Martínez, F.; Jouyban, A.; Acree,W. E. Some numerical analyses on the solubility of vanillin in Carbitol® + water solvent mixtures. Rev. Colomb. Quim. 2015, 44(2), 34-39. DOI: http://dx.doi.org/10.15446/rev.colomb.quim.v44n2.55218
Recibido: 10 de mayo de 2015. Aceptado: 25 de mayo de 2015
Abstract
In this communication some reported solubility values of vanillin (component 3) in 2-(2-ethoxyethoxy)ethanol (Carbitol®, component 1) + water (component 2) mixtures at five temperatures from 298.15 to 318.15 K were correlated with the Jouyban-Acree model combined with van't Hoff or Apelblat equations, obtaining models in second degree regarding the mixtures compositions. Mean percentage deviations were near to 6.0%. On the other hand, by means of the inverse Kirkwood-Buff integrals it was demonstrated that vanillin is preferentially solvated by water in water-rich mixtures (with a minimum δx1,3 value in the mixture x1 = 0.05, i.e. -4.29 x 10-2) but preferentially solvated by the cosolvent in mixtures with compositions 0.12 < x1 < 1.00 (with a maximum δx1,3 value equal to 3.61 x 10-2 in the mixture x1 = 0.25). It is conjectural that hydrophobic hydration plays a relevant role in the first case, whereas, in the second case, vanillin would be acting as Lewis acid with Carbitol®.
Keywords: Vanillin, Carbitol® + water mixtures, Jouyban-Acree model, preferential solvation, IKBI.
Resumo
Nesta pesquisa alguns valores de solubilidade da vanilina (componente 3) em misturas 2-(2-etoxietoxi)etanol (Carbitol®, componente 1) + água (componente 2) em várias temperaturas (298,15-318,15 K) foram correlacionados com o modelo Jouyban-Acree combinado com as equações do van't Hoff ou Apelblat obtendo modelos de ordem dois. Os desvios médios percentuais foram cercanos a 6,0%. Por outro lado, por meio das integrais inversas do Kirkwood-Buff demonstrou-se que a vanilina é preferencialmente solvatada pela água em misturas ricas em agua (com um valor mínimo de δx1,3 igual a -4,29 x 10-2 obtido na mistura de composição x1 = 0,05), mas, preferencialmente, solvatada pelo cosolvente, em misturas com composições 0,12 < x1 < 1,00 (com um valor máximo de δx1,3 igual a 3,61 x 10-2 obtido na mistura de composição x1 = 0,25). É conjecturável que a hidratação hidrofóbica desempenha um papel relevante no primeiro caso, enquanto que, no segundo caso a vanilina está atuando como ácido de Lewis com moléculas do Carbitol®.
Palavras chave: vanilina, misturas Carbitol® + água, modelo Jouyban-Acree, solvatação preferencial, IKBI.
Resumen
En esta comunicación se presenta la correlación de algunos valores de solubilidad de vainillina (componente 3) en mezclas 2-(2-etoxietoxi)etanol (Carbitol®, componente 1) + agua (componente 2) reportados previamente en la literatura a cinco temperaturas desde 298,15 hasta 313,15 K mediante el modelo de Jouyban-Acree combinado con las ecuaciones de van't Hoff y de Apelblat. En el análisis se obtuvieron modelos de segundo orden respecto a la composición de las mezclas disolventes. Las desviaciones porcentuales promedio fueron cercanas al 6,0%. Por otro lado, mediante las integrales inversas de Kirkwood-Buff se demostró que la vainillina es solvatada preferencialmente por el agua en mezclas ricas en agua (con un valor mínimo de δx1,3 igual a -4,29 x 10-2 obtenido en la mezcla de composición x1 = 0,05) pero preferencialmente solvatada por el cosolvente en mezclas con composiciones 0,12 < x1 < 1,00 (con un valor máximo de δx1,3 igual a 3,61 x 10-2 obtenido en la mezcla de composición x1 = 0,25). Se podría conjeturar que la hidratación hidrofóbica juega un papel relevante en el primer caso, mientras que en el segundo caso, la vainillina estaría actuando como ácido de Lewis frente a las moléculas de Carbitol®.
Palabras clave: vainillina, mezclas Carbitol® + agua, modelo Jouyban-Acree, solvatación preferencial, IKBI.
Introduction
Solubilization and desolubilization of solutes are required in many industrial applications. Despite of collecting experimental solubility data, mathematical models could be employed to provide predictive tools and also derive some thermodynamic parameters for better understanding of the phenomenon. Vanillin (Figure 1, 4-hydroxy-3-methoxybenzaldehyde, CAS number 121-33-5) is a flavoring agent commonly used in several industrial foods and pharmaceutical dosage forms as well as a fragrance in some cosmetic products (1,2). Carbitol® or Transcutol® (also known as 2-(2-ethoxyethoxy)ethanol, CAS number 111-90-0) is a commonly used cosolvent in some pharmaceutical and industrial formulations (1). Sullivan et al. (3) reviewed the safety of Carbitol® as a pharmaceutical excipient. In a recent paper, Shakeel et al. (4) reported the experimental solubility of a flavoring agent, vanillin (component 3), in some Carbitol® (component 1) + water (component 2) mixtures at different temperatures from 298.15 to 318.15 K along with some numerical correlation analyses. The work provided useful data for food industries and also expanded the available solubility database of the solutes in mixed solvents (5). The aim of this manuscript is to expand the results of numerical analyses in terms of the solubility data modeling according to the Jouyban-Acree model (6), as well as the evaluation of the preferential solvation of vanillin by both solvents in the saturated mixtures based on the inverse Kirkwood-Buff integrals (7). These complementary analyses provide trained versions of the cosolvency models for accurate prediction of vanillin solubility in Carbitol® + water mixtures at different temperatures. Otherwise, the preferential solvation analysis allows the proposal of mechanisms involved in the solubilization increasing by the cosolvents in aqueous mixtures. In turn, this research expands the analyses made previously with vanillin in 1,2-propanediol + water mixtures (8,9).
Results and discussion
The solubility data of vanillin at various temperatures was mathematically represented by both the van't Hoff and Apleblat equations (4). These Equations are represented as:
and
where x is the mole fraction solubility, T is the absolute temperature of the solution, a, b, A, B and C are the models constants. Equation [2] describes the solubility of vanillin in Carbitol®, water, and their mixtures more accurately than Equation [1], since it possess one more curve-fitting parameter. The overall mean percentage deviations (MPD) of 2.4 and 0.6 % were obtained from Equations [1] and [2], respectively, in which the MPD difference was statistically significant (paired t-test, p < 0.0004). The MPD was calculated by:
where N is the number of experimental data points and Xcal is the correlated solubility. There are some physicochemical reasons for observing these deviations from the linear pattern which are discussed by Grant et al. (10).
Shakeel et al. (4) used the log-linear model to represent the solubility of vanillin in the binary solvent mixture as:
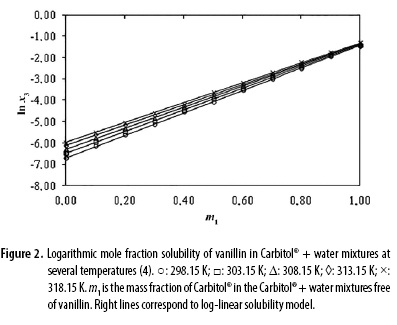
In which m1 and m2 are the mass fractions of Carbitol® and water in the absence of vanillin, X1,T and X2,T are the solubility of the solute in the mono-solvents 1 and 2 at T, i.e. in neat Carbitol® and water. The respective linear solubility trends at each temperature are shown in Figure 2.
Log-linear model is the simplest cosolvency model and is a popular equation in the pharmaceutical area, but it represents the effect of only solvent composition on the solubility and for each temperature a separate model should be trained. To cover these points and also to add the temperature term in the computations, the Jouyban-Acree model was derived by introducing additional terms to Equation [4] (6,11). The basic Jouyban-Acree model for representing the solubility of solutes in binary solvent mixtures at various temperatures is:
Where the Ji terms are the model constants computed using a no intercept least square analysis (12). The trained version of Equation [5] for vanillin solubility data in Carbitol® + water mixtures at various temperatures is:
Which back-calculated the solubility data with MPD of 5.6%. One may replace ln x1,T or ln x2,T with van't Hoff values as:
The J terms of Equation [7] are taken from Equation. [6] and the a and b terms from the corresponding van't Hoff analysis in neat solvents 1 and 2 and the provided model is:
The solubility of vanillin in these solvent mixtures at various temperatures was predicted using Equation [8] with the MPD of 6.0%. One may combine Equations [2] and [5]to provide the following model:
Which predicts the solubility data with the MPD of 5.7%. The main advantage of Equations. [8] or [9]over [6]is that these models do not require any further experimental data for predicting the solubility of vanillin in Carbitol® + water mixtures at various temperatures, whereas Equation [6] requires x1,T and x2,T values at each temperature of interest.
Furthermore, the preferential solvation parameter of vanillin by Carbitol® in Carbitol® + water mixtures (δx1,3) is defined as the following equation (13):
where, XL1,3 is the local mole fraction of Carbitol® in the environment near to vanillin. If δx1,3 > 0 vanillin is preferentially solvated by Carbitol®. If this parameter is negative vanillin is preferentially solvated by water. δx1,3 values are obtained from the inverse Kirkwood-Buff integrals for the individual solvent components as shown in Equations [11] and [12]:
Where κT is the isothermal compressibility of the solvent mixtures (in GPa-1), V1 and V2 are the partial molar volumes of the solvents, and V3 is the partial molar volume of vanillin. The functions D and Q are defined by the following equations:
Here, 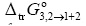 is the standard molar Gibbs energy of transfer of vanillin from neat water to Carbitol® + water mixtures and
is the standard molar Gibbs energy of transfer of vanillin from neat water to Carbitol® + water mixtures and 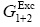 is the excess molar Gibbs energy of mixing of Carbitol® and water in the mixtures free of vanillin.
is the excess molar Gibbs energy of mixing of Carbitol® and water in the mixtures free of vanillin.
With all these quantities the preferential solvation parameter was calculated from the Kirkwood-Buff integrals as follows:
To use Equation [15], the correlation volume (Vcor) was obtained by means of the following expression:
Where r3 is the molecular radius of vanillin (expressed in nm). However, the definitive correlation volume required iteration done by replacing δx1,3 in the Equation [10] to calculate XL1,3 until a non-variant value of Vcor is obtained.
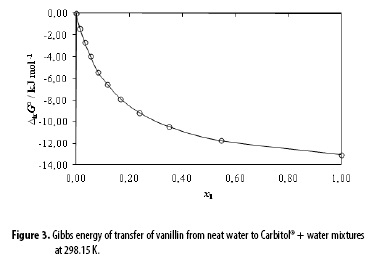
Figure 3 shows the Gibbs energy of transfer behavior of vanillin from neat water to Carbitol® + water mixtures at 298.15 K. These values were calculated from the mole fraction solubility of vanillin reported by Shakeel et al. (4) according to the following equation:
The 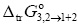 values were correlated with the polynomial presented as Equation [18] with the following coefficients (kJ mol-1): α = 0.00, b = 1.75, c = -143.95, d = 338.85, e = 320.79 and f = 111.10, with r2 = 0.9998 and standard fitting error = 0.0608.
values were correlated with the polynomial presented as Equation [18] with the following coefficients (kJ mol-1): α = 0.00, b = 1.75, c = -143.95, d = 338.85, e = 320.79 and f = 111.10, with r2 = 0.9998 and standard fitting error = 0.0608.
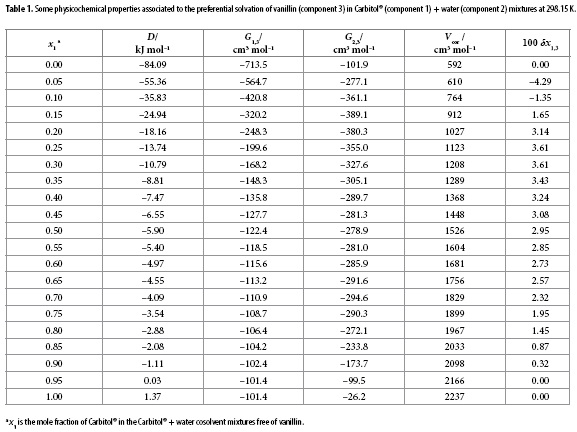
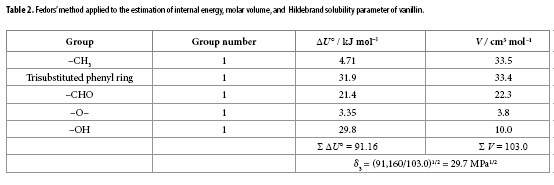
In this way, the D values reported in Table 1 were calculated from the first derivative of the polynomial model solved according to the mixture's composition. The values of Q, RT κT and partial molar volumes of the solvents in the mixtures were taken from the literature (14). Molar volume of vanillin was calculated by means of the Fedors' method (15) as shown in Table 2. G1,3 and G2,3 values shown in Table 1 are negative in all cases indicating that vanillin exhibits affinity for both solvents in the mixtures, i.e. Carbitol® and water. Solute radius value (r3) was also calculated from the vanillin molar volume as 0.344 nm according to that described earlier (9). The correlation volume of this compound was iterated three times by using Equations [10], [15] and [16] to obtain the values reported in the Table 1. This table also shows the preferential solvation parameters of vanillin by Carbitol®.
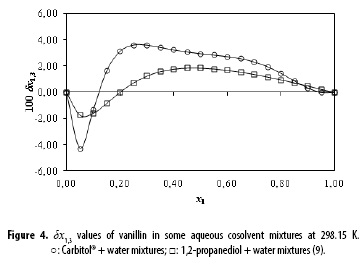
Table 1 and Figure 4 show that the δx1,3 values vary non-linearly with the Carbitol® proportion in the aqueous mixtures. Thus, the addition of Carbitol® to water makes negative the δx1,3 values of vanillin from neat water to the mixture x1 = 0.12 reaching a minimum value in the mixture x1 = 0.05 with δx1,3 = -4.29 x 10-2. It would be possibly that the hydrophobic hydration around the non-polar groups of vanillin contributes to lower the net δx1,3 to negative values in these water-rich mixtures (9).
In mixtures with composition 0.12 < x1 < 1.00, the local mole fractions of Carbitol® are higher than those in the bulk mixtures because δx1,3 are positive. In this way, the cosolvent action of Carbitol® to increase the vanillin solubility could be related to the breaking of the ordered structure of water around the non-polar moieties of vanillin. This increases the solvation by Carbitol® exhibiting a maximum value in the mixture x1 = 0.25 with δx1,3 = 3.61 x 10-2. It is conjecturable that in the 0.12 < x1 < 1.00 region vanillin is acting as a Lewis acid with Carbitol® molecules because this cosolvent would be more basic than water owing their ether and hydroxyl groups (Figure 1) (14).
Finally, Figure 4 also compares the preferential solvation of vanillin in Carbitol® + water and 1,2-propanediol + water mixtures (9). Clearly the magnitude of preferential solvation of vanillin by cosolvent and water is higher in Carbitol® + water mixtures compared with 1,2-propanediol + water mixtures. It is important to note that Carbitol® is less polar than 1,2-propanediol as described by their Hildebrand solubility parameters, i.e. δ = 22.3 MPa1/2 for Carbitol® and 30.2 MPa1/2 for 1,2-propanediol (16). This behavior is similar to that exhibited by the antioxidant agent daidzein and the analgesic drug ketoprofen in ethanol + water and 1,2-propanediol + water mixtures. It is noteworthy that ethanol is less polar than 1,2-propanediol (17,18). In turn, the composition interval lengths regarding vanillin are contrary when compared with daidzein and ketoprofen. Thus, the composition interval, where vanillin is preferentially solvated by water in Carbitol® + water mixtures, is lower than that for the same compound in 1,2-propanediol + water mixtures. This result is opposite to that reported for daidzein and ketoprofen in ethanol + water and 1,2-propanediol + water mixtures. In this way, the composition regions where the drugs are preferentially solvated by water in ethanol + water mixtures are higher than those for the same compounds in 1,2-propanediol + water mixtures. Unfortunately, the molecular reasons involved in these opposite behaviors are not clear because the complexity of the molecular structures of the compounds under analysis. Nevertheless, it is noteworthy that the observed and discussed preferential solvation of vanillin in Carbitol® + water mixtures is in very good agreement with that previously described based on more classical thermodynamic treatments (4).
Conclusions
Published vanillin solubility values in Carbitol® + water mixtures have adequately been correlated with the Jouyban-Acree model combined with van't Hoff and Apelblat equations. Vanillin is preferentially solvated by water in water-rich mixtures, but preferentially solvated by the cosolvent in mixtures with compositions 0.12 < x1 < 1.00. Based on these behaviors, it is conjectural that hydrophobic hydration plays a relevant role in the first case, whereas, in the second case, vanillin would be acting as Lewis acid with Carbitol® molecules.
References
1. Budavari, S.; O'Neil, M. J.; Smith, A.; Heckelman, P. E.; Obenchain, J. R., Jr.; Gallipeau, J. A. R.; D'Arecea, M. A. The merck index, an encyclopedia of chemicals, drugs, and biologicals, 13th ed. Merck & Co., Inc.: Whitehouse Station, NJ, 2001; pp 1768-1769. [ Links ]
2. Rowe, R. C.; Sheskey, P. J.; Owen, S. C. (eds.). Handbook of pharmaceutical excipients, 5th ed. Pharmaceutical Press and American Pharmacists Association: Grayslake, IL, 2006; pp 798-799. [ Links ]
3. Sullivan, D. W., Jr.; Gad, S. C.; Julien, M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol ether (DEGEE) used as a pharmaceutical excipient. Food Chem. Toxicol. 2014, 72, 40-50. DOI: http://dx.doi.org/10.1016/j.fct.2014.06.028. [ Links ]
4. Shakeel, F.; Haq, N.; Siddiqui, N. A.; Alanazi, F. K.; Alsarra, I. A. Solubility and thermodynamics of vanillin in Carbitol-water mixtures at different temperatures. LWT - Food Sci. Technol. 2015, 64, 1278-1282. DOI: http://dx.doi.org/10.1016/j.lwt.2015.07.043. [ Links ]
5. Jouyban, A. Handbook of solubility data for pharmaceuticals. CRC Press: Boca Raton, FL, 2010; pp 203-505. [ Links ]
6. Jouyban-Gharamaleki, A.; Acree, W. E., Jr. Comparison of models for describing multiple peaks in solubility profiles. Int. J. Pharm. 1998, 167, 177-182. DOI: http://dx.doi.org/10.1016/S0378-5173(98)00073-8. [ Links ]
7. Marcus, Y. Solvent mixtures: Properties and selective solvation. Marcel Dekker, Inc.: New York, 2002; pp 180-238. [ Links ]
8. Jouyban, A.; Acree, W. E., Jr. Comments on "Solubility and thermodynamic behavior of vanillin in propane-1,2-diol + water cosolvent mixtures at different temperatures''. Food Chem. 2016, 192, 1049-1050. DOI: http://dx.doi.org/10.1016/j.foodchem.2015.07.120. [ Links ]
9. Martínez, F.; Jouyban, A.; Acree, W. E., Jr. Further comments on "Solubility and thermodynamic behavior of vanillin in propane-1,2-diol + water cosolvent mixtures at different temperatures''. Food Chem. 2016, 196, 757-759. DOI: http://dx.doi.org/10.1016/j.foodchem.2015.10.017. [ Links ]
10. Grant, D. J. W.; Mehdizadeh, M.; Chow, A. H. -L.; Fairbrother, J. E. Nonlinear van't Hoff solubility-temperature plots and their pharmaceutical implications, Int. J. Pharm. 1984,18, 25-38. DOI: http://dx.doi.org/10.1016/0378-5173(84)90104-2. [ Links ]
11. Acree, W. E., Jr. Mathematical representation of thermodynamic properties. Part II. Derivation of the combined nearly ideal binary solvent (NIBS)/Redlich-Kister mathematical representation from a two-body and three-body interactional mixing model. Thermochim. Acta. 1992, 198, 71-79. DOI: http://dx.doi.org/10.1016/0040-6031(92)85059-5. [ Links ]
12. Jouyban-Gharamaleki, A.; Hanaee, J. A novel method for improvement of predictability of the CNIBS/R-K equation. Int. J. Pharm. 1997, 154, 245-247. DOI: http://dx.doi.org/10.1016/S0378-5173(97)00136-1. [ Links ]
13. Marcus, Y. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 2008, 140, 61-67. DOI: http://dx.doi.org/10.1016/j.molliq.2008.01.005. [ Links ]
14. Martínez, F.; Jouyban, A.; Acree, W. E., Jr. Comments on "Solubility and thermodynamic function of a new anticancer drug ibrutinib in 2-(2-ethoxyethoxy)ethanol + water mixtures at different temperatures". J. Chem. Thermodyn. 2016, 95, 180-182. DOI: http://dx.doi.org/10.1016/j.jct.2015.11.031. [ Links ]
15. Fedors, R. F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147-154. DOI: http://dx.doi.org/10.1002/pen.760140211. [ Links ]
16. Barton, A. F. M. Handbook of solubility parameters and other cohesion parameters, 2nd ed. CRC Press LLC: Boca Raton, FL, 1991; pp 103, 116. [ Links ]
17. Jiménez, D. M.; Cárdenas, Z. J.; Delgado, D. R.; Peña, M. Á; Martínez, F. Preferential solvation of the antioxidant agent daidzein in some aqueous co-solvent mixtures according to IKBI and QLQC methods. J. Appl. Sol. Chem. Model. 2015, 4, 110-118. DOI: http://dx.doi.org/10.6000/1929-5030.2015.04.02.3. [ Links ]
18. Cárdenas, Z. J.; Jiménez, D. M.; Martínez, F. Preferential solvation of ketoprofen in some co-solvent binary mixtures. J. Solution Chem. 2014, 43, 1904-1915. DOI: http://dx.doi.org/10.1007/s10953-014-0255-3. [ Links ]