Introduction
Rosaceae family consists of a great variety of plants with edible fruits, healthy and of great economic importance such as apple, peach, pear, quince, loquat, etc. [1]. The annual production of these fruits in the world is of millions of tons. In Peru, the production of apples, peaches/nectarines, pears, quinces, and loquats reach a total of 158, 51, 4, 7 and 2 thousand tons, respectively [2]. The surface of the fruits is covered by a lipophilic layer called cuticle, which has two main components: (i) the lipophilic compounds released by solvent extraction which are collectively designated as cuticular wax and (ii) the lipophilic component that cannot be extracted due to its polymer structure which is called cutin [3]. Cutin is a polyester-type biopolymer, composed mainly of fatty acids [4], while cuticular wax is typically a mixture of dozens of compounds with a variety of hydrocarbon chains or conjugated rings, including pentacyclic triterpenes [5].
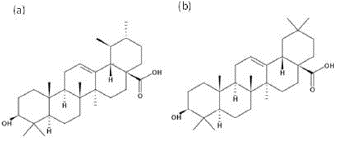
Ursolic acid (UA) and oleanolic acid (OA) (Fig. 1) are two of the main pentacyclic triterpenes found in the cuticular wax of many fruits, vegetables, and plants [5, 6]. These molecules are of great interest because they have been shown to possess anti-oxidant, anti-microbial, anti-inflammatory, gastroprotective, and analgesic. [7-12].
The objective of the present work is to determine the content of UA and OA in the cuticular wax of fruits from plants belonging to the Rosaceae family (apple, peach, pear, quince and loquat) grown in Cusco, Peru.
Materials and methods
Materials and Reagents
Five fruits: apple (Malus domestica), peach (Prunus persica), pear (Pyrus communis), quince (Chaenomeles japónica), and loquat (Eriobotrya japónica) were purchased at the SanPedro, Cusco-Peru market and transported directly to the laboratory. Fruits (250 g) were washed with tap water before removing the peel from the fruits. The peels were then dried at 80 °C for 12 h, which were crushed in a porcelain mortar and kept in a desiccator until being analyzed. The chemical reagents used, ethanol (Merck, Germany) and acetonitrile (Merck, Germany), were chromatographic grade, and methanol (Merck, Germany), petroleum ether 40-60 °C (Merck, Germany) together with chloroform (Merck, Germany) were of analytical grade.
Cuticular wax extraction
The extraction of cuticular wax is more efficient when using a mixture of CHCl3: MeOH (3:1) [13]. Therefore, the dried and crushed apple (1.04 g), peach (1.01 g), pear (1.00 g), quince (1.01 g), and loquat (1.03 g) peels were immersed in 10 mL of the solvent mixture, and then, the suspensions were extracted with ultrasound assistance under 20 °C for 3 min.
This process was repeated three times. The extracts were collected and filtered to be concentrated by evaporating at 60 °C and 230 mbar (reduced pressure) in a rotary evaporator. The concentrates were defatted by washing three times with 15 mL of petroleum ether (5 mL each time), then made up to 25 mL with ethanol. Finally, all the extracts were filtered through a 0.45 μιη membrane and stored at 4 °C for subsequent chromatographic analysis.
Thin layer chromatography
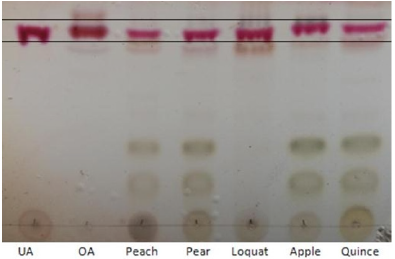
Thin layer chromatography was performed on a silica gel 60-F254 plate with 10-12 μιη particle size. Standard solutions of UA (1 mg/mL), OA (1 mg/ mL) and test solutions were applied on silica gel plate and developed with a mixture of solvent CHCl3: MeOH (12:2 v:v). After developing and drying, the plate was dipped for 2 s in sulfuric acid (10% v/v) and heated on a plate heater. Triterpene acids are shown as reddish colored areas [14].
Determination of the content of UA and OA
The quantification of OA and UA was performed by HPLC, for which an UltiMate 3000 chromatograph with DAD detector (Thermo, USA), Lichrospher RP-18 column (250 x 4.6 mm, 5 μΓη) was used, detection was performed at 209 nm, temperature of 30 °C with a flow of 1 mL/min. The mobile phase consisted of a mixture of acetonitrile:water (8:2 v:v) with an isocratic elution mode [15]. Mixtures of UA and OA (0.5 mg/mL) were prepared in ethanol and volumes of 1, 0.5, and 0.1 μL injected for plotting the standard curves for UA and OA. The injection volume of the samples was 1 μL. The calibration curves for the determination of UA and OA were constructed and the following Ec. (1) and Ec. (2) were obtained.
Where Y is the peak area (mAU S) and X is the amount (μ g). The correlation coefficient (R 2 ) of the regression equation of OA and UA was 0.99 for both curves.
Result and discussion
Analysis of thin layer chromatography
An image of the chromatographic plate of the standards and sample test is shown in Figure 2. OA and UA travel at same rates in the chromatographic plate because of the closely related physicochemical properties of both structural isomers. The coloration that was acquired after being revealed with sulfuric acid and submitted to the heat is reddish as expected. The presence of these triterpenes can be visualized in all the samples.
UA and OA contents in the cuticular wax of fruits
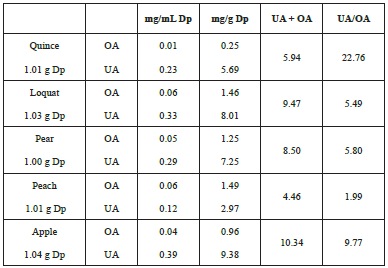
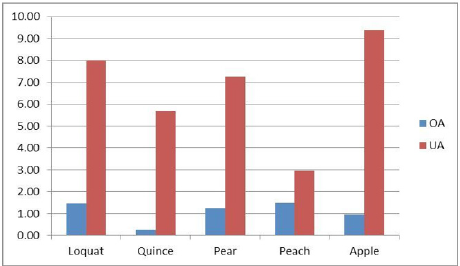
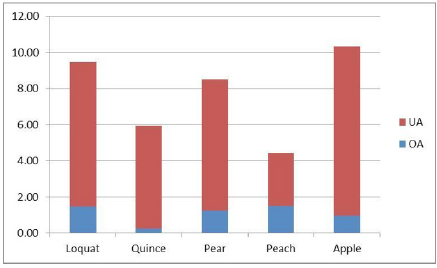
The results of the chromatographic analysis (Fig. 3) show that OA and UA acid were the main two active compounds in the skin of these five edible fruits. The concentration of UA ranged from 2.97 to 9.38 mg/g and that of OA from 0.25 to 1.49 mg/g (Table 1). The content of UA was significantly greater than that of OA in all cases (Fig. 4). The total content of these two triterpenes (UA+OA) is higher in the apple (10.34 mg/g) and lower in the peach (4.46 mg/g) (Fig. 5). The proportion of these two triterpenes (UA/ OA) is higher in the quince (22.76) and less in the peach (1.99).
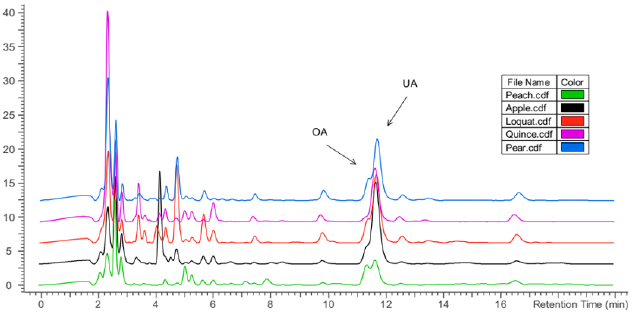
Figure 3 Chromatograms of the five samples, which were obtained using a mobile phase of acetonitrile: water (8:2, v:v) with a flow of 1 mL/min and detection at 209 nm.
The content of UA and OA in loquat was not significant different from those obtained by Zhou et al. [ 16]. Yin et al. [ 13] identified a series of pentacyclic triterpenes in the polar fraction of the cuticular wax of the pear, but does not give information about their concentrations of these or the type of triterpene present. Comparing the content of OA and UA in the quince peel determined by Miao et al. [ 17] and our results, the levels of UA and OA were higher in this research. Consistent with the work of Belgeel et al. [ 18], OA and UA were the main triterpenes in peach peel. However, unlike the other fruits where the UA is the triterpene in greater quantity, in the peach the difference between the amounts of OA and UA is low.
The amount that was extracted from UA and OA of the apple peel was higher by the method described in this work than the one proposed by Ellgardt [19].
Conclusions
In this study, the content of UA and OA present in the cuticular wax of the five most edible fruits (quince, loquat, pear, peach and apple) of the family Rosaceae was determined. Ursolic and oleanolic acid were the predominant triterpenes in the cuticular wax of these fruits, and the UA content were higher than OA in all samples.