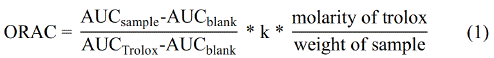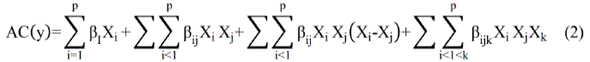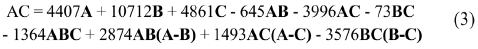Introduction
The cocoa plant (Theobroma Cacao L.) is a native species from South and Central America. It is classified into three varieties: Criollo, Forastero, and Trinitario [1,2]. The high content of micronutrients, macronutrients, and bioactive compounds presents in cocoa beans, justify their importance in the food, cosmetic, and pharmacological industries [3,4]. In fact, cocoa beans hold bioactive compounds, such as polyphenols, which have antioxidant, anticancer and antimicrobial properties tested at in vitro and in vivo levels [5,6]. These compounds are reflected in positive health effects; for example, prevention of cardio and cerebrovascular diseases, reduction of cholesterol levels, improvement in endothelial dysfunction, and treatment in some neurodegenerative diseases [5,6].
Cocoa is ranked fourth among the 100 richest sources of polyphenols, surpassed only by clove, spearmint, and anise [7]. However, the last three are seasonal spices, only extensively used in a few countries, which makes cocoa the richest food in polyphenols consumed massively around the world.
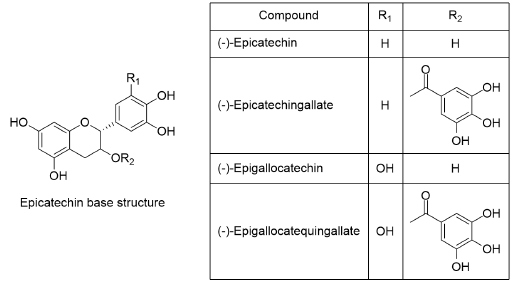
Polyphenols in cocoa beans are part of a chemical family known as flavonoids, flavan-3-ol or catechin, they are also present in other fruits such as apple, tea and wine[8]. Cocoa bean catechins are formed by a base structure accompanied by different functional groups, which give them different physicochemical properties. Within this family, there are molecules such as (+)-catechin, (-)-epicatechin, epicatechin-gallate, epigallocatechin and catechin-gallate, whose degree of polymerization (DP) value is 1 (Figure 1) [9,10]. In addition, the union of these monomers creates larger size oligomers (DP value ≥ 2) [11].
Several studies account for various polyphenol extraction methods from vegetable materials using conventional and non-conventional techniques. Conventional extraction technologies include infusion, solid-liquid extraction, liquid-liquid extraction, solid phase separation, among others [10,12-15]. Non-conventional technologies are microwave-assisted extraction, supercritical fluids, and ultrasonic waves [16,17]. In general, most of these studies aimed at finding the best conditions to obtain the highest extraction yield as well as identifying GRAS degree solvents that soon would expand the use of polyphenol extracts in food, cosmetics, or pharmaceutical applications [18].
Despite the current knowledge about the chemical profile of compounds present in polyphenol extracts obtained from cocoa beans, the role of each chemical family within the extract, from an antioxidant point view, is not entirely understood. For example, Toro et al. [19] evaluated the effects of cocoa polyphenol extracts and their different isolated fractions on the antioxidant capacity measured by ORAC, DPPH, and ABTS methods. According to their results, the crude extract had the best antioxidant capacity, compared to the individual fractions. For example, the antioxidant activity, (AA), measured by ORAC for the crude extract was 50.16 ± 1.72; while for catechin and epicatechin (monomers) it was 13.7 ± 1.0 and 13.0 ± 0.5, respectively. For fraction I (mainly dimer) the AA was 16.6 ± 0.2, for fraction II (mainly trimer) 15.4 ± 0.6, for fraction III (mainly tetramer) 13.9 ± 0.7 and for fraction IV (mainly hexamer) 12.4 ± 1.0. The authors concluded that improved antioxidant capacity in crude extract could result from the synergic interaction of fractions and monomers. However, a mathematical model to confirm that synergism exists still needs to be developed.
Deprez et al. [20] reported that only dimers and trimers of flavonols were bioaccessible and bioavailable in the digestive tract, due to weight and molecular size. Also, Pérez-Jimenez [7] and Rahman [21] noted that at in vitro level, molecules with more hydroxyl functional groups hold greater antioxidant capacity; however, this is a dynamic behavior. Depending on the flavonoid DP value and its functional groups, there are synergistic or antagonistic effects over the antioxidant capacity, but it is still a challenge to be confirmed. Therefore, the aim of this paper was to propose a proper statistical treatment to describe the quantitative relationship between antioxidant capacity and polymerization degree using different fractions obtained from cocoa bean extracts.
Materials and methods
Chemical reagents
Ascorbic acid, citric acid, 2,2'-azobis(2-amidinopropane), dihydrochloride (AAPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox®), and L-cysteine were obtained from Sigma-Aldrich. Analytical grade ethanol, Folin-Ciocalteu phenol reagent, gallic acid, and sodium carbonate were obtained from Merck.
Raw material
Forty cocoa pods (clone ICS-39) were obtained from the experimental farm Villa Juliana, located in Rionegro, Santander, Colombia (average altitude: 590 masl, average temperature: 25 °C and average relative humidity: 87%). All fruits were harvested with homogeneous maturity and similar morphological characteristics. Afterward, fresh cocoa beans were carefully and manually separated from the husk to avoid damage by cuts. Pulp and mucilage were manually removed from unfermented cocoa beans.
Pretreatment of cocoa beans
The beans were subjected to a combined thermochemical process to avoid polyphenols' oxidation by the polyphenol oxidase (PPO) enzyme, as described by Toro et. al. [19]. In brief, unfermented cocoa beans were placed in a solution of 70 mM ascorbic acid/L-cysteine (1:1, v/v) at 96 °C for 6.4 min. Then, cocoa beans were submerged in ice water for 30 min. After that, the beans were washed three times with distilled water (4 °C) to remove traces of ascorbic acid and L-cysteine. The cocoa beans were chopped by hand (cross-section of 50 x 30 mm2) with a knife and immediately oven-dried (Binder model FD 23, Tuttlingen, Germany) at 70 °C for 3 h (final water content < 4%). After drying, the samples were cooled to -20 °C, milled (Grindomix GM 200, Retsch GmbH, Haan, Germany) three times for 30 s, and then sieved through a 120-mesh screen (W.S. Tyler, Mentor, OH). Finally, the milled cocoa beans were stored in the dark at -20 °C until subsequent extraction of polyphenols.
Bromatological characterization of cocoa beans
Unfermented cocoa beans were characterized by using methods of the Official Analytical Chemist Association as follows: moisture 966.02, ash 923.03, fat 920.39, fiber 920.87 and proteins 962.09. Carbohydrates were calculated from the above results.
Extraction of polyphenols
Solid-liquid extraction of polyphenols was done using the methodology proposed by various authors [18,19] with some modifications. The milled cocoa beans obtained were mixed with an ethanol:water solution (1:1 v/v) using a mass:solvent ratio of 1:120 (w:v). Then, this mixture was placed in an ultrasound bath (Ultrasonic LC 30H, Elma, Singen, Germany) at 20 kHz and 4°C for 15 min. Subsequently, the solution was acidified to pH 6.0 using citric acid (10 mM) and the extraction process continued for 30 min at 70 °C. Afterward, the extract was centrifuged at 4000 rpm for 15 min at 4 °C. Then, the supernatant was collected and filtered with Whatman filter paper N° 1. The ethanol was removed by vacuum evaporation (55 °C and 13,500 Pa). The crude extract was then freeze-dried (primary drying at -40 °C and 13000 Pa for 36 h and secondary drying in a gradient from -10 °C to 45 °C for 24 h). Finally, the lyophilized extracts were refrigerated (-80 °C) and stored in the dark for further analysis.
Total polyphenolic content (TPC)
The TPC was determined following the procedure described by various authors [3,10,14,18]. In sum, 50 µL of polyphenol extract were mixed with 1.5 mL of Folin-Ciocalteu phenol reagent (10% v/v) and incubated for 5 min in darkness. Then, 1.5 mL of sodium carbonate (7.5% w/v) were added to mixture as the reaction continued in darkness during 60 min. The absorbance of the samples was measured at 765 nm, using distilled water as blank. Finally, TPC was determined and expressed as mg of gallic acid equivalents per g of dry sample (mg GAE/g ds).
Antioxidant capacity
The antioxidant capacity of the polyphenols extract was determined by the oxygen radical absorbance capacity (ORAC) assay, following the methodology proposed by Huang et al. [22]. In short, ninety-six well microplates were filled with 150 µL of the daily-working fluorescein solution (8.16 • 10-5 M in 75 mM phosphate buffer, pH 7.4) and 25 µL of polyphenol extract at a known concentration. Then, they were incubated at 37 °C for 10 min in a microplate reader (Synergy HT Multi-Detection, Biotek Instruments, Inc. Winooski, VT, USA). The reaction was initiated by the addition of 25 µL of AAPH solution (153 mM in 75 mM phosphate buffer, pH 7.4). Then, the fluorescence decay was kinetically monitored, every minute during 1 h, using emission and excitation wavelengths of 485 and 528 nm, respectively. A calibration curve was prepared with 3-25 µmol Trolox per g of cocoa beans (dry matter basis) as indicated by Eq. (1) (R2 = 0.99).
Where k is the sample dilution factor, and AUC is the area below the fluorescence decay curve of the sample, blank, and Trolox, respectively. The area under the curve of normalized data was calculated using GraphPad Prism v.6.0 (GraphPad Soft. Inc., La Jolla, California, USA).
Identification, separation, and quantification of main groups of polyphenols using HPLC/UV-vis and HPLC/MS
This section describes the method used to identify, separate, and quantify fractions with different degree polymerization of: (i) monomers, (ii) dimers and (iii) oligomers present in the extract. In order to reach this objective, the method described by Toro-Uribe et al. [19] was used. The chromatographic separation was developed in a Thermo Scientific UltiMate 3000 HPLC, equipped with an auto-injection system and UV-VIS detector (Thermo Scientific VWD-3100). A reverse-phase analytical column (Zorbax Eclipse XDB C18 150 mm X 2.1 mm X 5 µm) and a semipreparative column (Mediterranea Sea 150 mm X 4.6 mm X 5 µm) were used. The column was heated at 40 °C using a furnace (Thermo Scientific TCC-3000 SD). All compounds were detected at 204 nm. The injection volume was 20 µL for the analytical column and 200 µL for the semipreparative column. The mobile phase consisted of 0.1% (v/v) formic acid (Solvent A) and acetonitrile (Solvent B) with a flow rate of 0.2 mL/min for the analytical column and 2 mL/min for the semipreparative column. The gradient was as follows: 0-20 min, 6-10% B; 20-25 min, 10-13% B; 25-30 min, 13-15% B; 30-40 min, 15-10% of B; 40-45 min, 10-6% B, followed by 10 min re-equilibration of the column before a new injection.
Identification of monomers, dimers, and oligomers was carried out using an Ion Trap LC/MS (model 6320, Agilent Technologies, Waldbronn, Germany) equipped with an ESI source and an ion trap mass analyzer, which was controlled by the 6300 series trap control software (Bruker Daltonik GmbH, V. 6.2). The mass spectrometer was operated under negative ESI mode with the following conditions: mass spectra recorded from 90-2200 m/z, nebulizer 40 psi, dry gas 12 L/min, and dry temperature 350 °C.
Finally, each chemical family was quantified using epicatechin as external standard. The results were expressed as mg epicatechin equivalent per gram of dry sample (mg EE/g ds).
Statistical analysis
Synergistic or antagonistic effects in the antioxidant capacity of fractions with different DP obtained from the cocoa bean extracts were evaluated with a simplex lattice design with three repications. The response variable of interest was the antioxidant capacity. This design allows us to fit the data into a complete cubic model, as shown in Eq. (2).
All measurements were done three times, and data were expressed as means ± standard deviations. Statistical analysis was done using the TIBCO Statistica software 13.5 Desktop Version 1.35.
Results and discussion
Bromatological characterization of cocoa beans
Results of bromatological analysis for cocoa beans after inactivation, dried, and sieved are reported in Table 1. These results showed that the values obtained in this work are in the same ranges of the values reported by FEDECACAO [23] for cocoa beans from different regions of Colombia. In the same way as described by Alvarez [24] and Vázquez-Ovando [25] for cocoa beans from Venezuela and Mexico, respectively.
Table 1 Composition of ICS-39 cocoa beans reported in this paper and other sources.
| Components | This work* | FEDECACAO | Venezuelan cocoa beans | Mexican cocoa beans |
|---|---|---|---|---|
| Moisture, % | 5.9 ±0.3 | 7.0 ±0.1 | 6.37 | 7.06 |
| Fat, (g/100 g dry sample) | 48 ±2 | 54.95 ±0.14 | 56.01 | 57.13 |
| Protein, (g/100 g dry sample) | 12 ± 1 | 15.06 ±0.90 | 14.00 | 18.54 |
| Ash, (g/100 g dry sample) | 3.2 ±0.1 | 3.54 ±0.50 | 3.32 | 7.03 |
| Fiber, (g/100 g dry sample) | 3.7 ±0.2 | 2.14 ±0.20 | 0.42 | NR |
| Carbohydrates, (g/100 g dry sample) | 36.0 ± 1.2 | 26.45 | 31.25 | 47.10 |
| Caloric value, (kcal/100 g) | 628.94 | 660.59 | 685.09 | 776.73 |
Total polyphenolic content
In the polyphenol extracts, total polyphenolic content (TPC) in the polyphenol extracts was quantified in 77 mg GAE/g ds; this value is higher than the reported for Colombian cocoa beans. For example, Peñaranda & Sierra [26] reported values between 49.6 and 74.47 mg GAE/g ds for ten cocoa materials introduced as well as the 11 most commercialized regional materials in Colombia. Alean [27] reported a TPC of 62.97 mg GAE/g ds for the cocoa beans (clone CCN-51) harvested in Colombia. Also, the TPC obtained in this work was higher than the reported by Yanzapanta [28] in Ecuador. In this case, average values were 43.47 and 71.66 mg GAE/g ds for fine cocoa (clones EET-62 and EET-103) and ordinary cocoa (clone CCN-51, EET-400 and ICS-95), respectively.
The higher value of total polyphenols reported in this research can be attributed to the cocoa clone as well as the inhibition of the PPO enzyme that reduces the decomposition of these compounds, since its action on cocoa beans is significantly reduced [19,29].
In any extraction process, it is vital to know the yield of the process for economic analysis and a possible scale-up. The yield of the extractions was calculated by relating the amount of lyophilized extract with the amount of mass charged for extraction, obtaining a value of 7 %. Similar yield values were reported by Pedan et al. [30], who quantified polyphenols for dried and fermented cocoa beans were from a genuine Trinitario variety grown and harvested in Costa Rica. It is important to highlight the extraction used in this work uses GRAS solvents (ethanol and water), which are ideal for scale-up in the food industry.
Antioxidant capacity
The antioxidant capacity (AC) for polyphenol extracts was determined by ORAC assay. The average value for AC obtained in this work was 3,478 µmol TE/g ds. This value was 1.2 times higher than what was reported by Zapata et al. [31], who used the same assay. Actually, Zapata et al. [31] founded that AC value for several cocoa beans (clones CCN-51, ICS 1, ICS 60, ICS 95 and TSH 565) were between 1,473.22 and 2,873.58 pinol TE/g ds. In contrast, Haytowitz & Bhagwat [32] made a report of antioxidant capacity for different matrices, including unsweetened cocoa powder, reporting a AC value of 556 µmol TE/g ds. The difference between the AC value obtained in this work could be explained considering that in the case of Zapata et al. [31] an inhibition treatment was not carried out; while in Haytowitz & Bhagwat [32] case, the difference may be attributed to the transformation of cocoa beans into cocoa powder, involving a process that drastically decreases the content of bioactive compounds.
Identification, separation, and quantification of main groups of polyphenols using HPLC/UV-vis and HPLC/MS
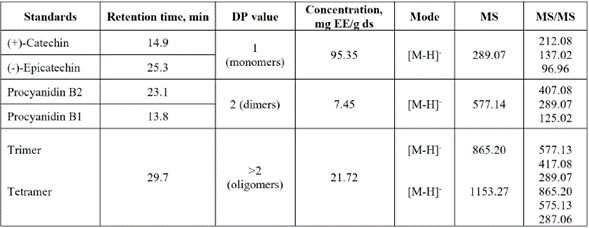
The separation of different chemical families presents in the extract was performed using HPLC preparative. Peaks in Figure 2 show that correspond to monomers ((+)-catechin and (-)-epicatechin), dimers (procyanidin B1 and procyanidin B2), oligomers, and methylxanthines (theobromine and caffeine). The identification of monomers, dimers, and methylxanthines was performed by comparison between the time retention peak of standards and the sample. Besides, the identity of oligomers with DP>2 was confirmed using mass spectrometry. The results of m/z ratio showed [M-H]- ions at m/z 865.2, 1153.3, 1442.3, and 1730.4 that correspond to trimer, tetramer, pentamer, and hexamer structures, respectively (Table 2).
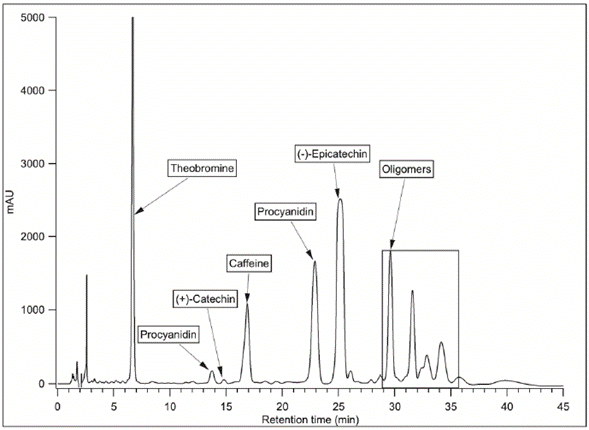
Figure 2 HPLC chromatogram of the different polyphenol fractions in ICS-90 cocoa bean extracts using conditions for the semipreparative column.
Polyphenolic content of specific DP value
Once the polyphenols were collected, according to DP value and retention times defined in the previous section, the polyphenolic compounds were checked by HPLC with an analytical column. It was corroborated that the extracts collected contain monomers, dimers, and oligomers only.
For each extract, the polyphenolic content was reported by using (-)-epicatechin as standard for quantification, in order to have a point of comparison between the collected extracts of monomers, dimers, and oligomers (Table 2). Due to the greater proportion of (-)-epicatechin presented in the extracts, it is a viable compound as a standard for the determination of polyphenolic content. (Figure 2). Also, other authors have quantified polyphenols as equivalent to (-)-epicatechin, such as Niemenak et al. [33], who made a comparative study of different cocoa beans in Cameroon, in terms of their content of phenolic compounds and anthocyanins.
Mix design of polyphenolic extracts of different DP value
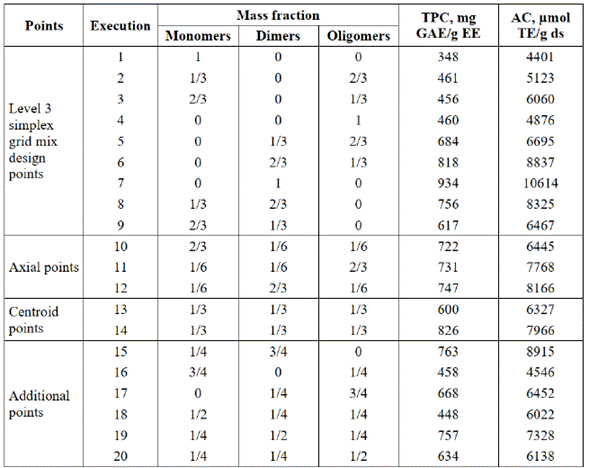
The AC was evaluated by mixing up to three components (monomers, dimers and oligomers). TPC was also determined for each of the mixing points. Additional points were performed to verify the model fit. The results were expressed as mg of gallic acid equivalents per g of epicatechin equivalent (mg GAE/g EE), Table 3.
To have a reference in the quantification of polyphenols in the purified extracts, TPC was determined for the standards (+)-catechin and (-)-epicatechin, two of the most abundant molecules in polyphenols of cocoa beans. The values obtained were 1036.98 and 665.29 mg GAE/g, respectively. Similar values were evidenced for the quantized mix design points. This suggests that, although the TPC values were higher as compared to the typical TPC values for plant matrices, they are real and comparable with the standards. This is due to the nature of the method. Since the Folin-Ciocalteu assay is based on redox reactions, there is the possibility that certain compounds of the complex plant matrix act as interference, leading to lower values of TPC because of the action of compounds with the ability to infer in redox reactions. In the case of purified and separated polyphenolic extracts of monomers, dimers, and oligomers, this event does not occur.
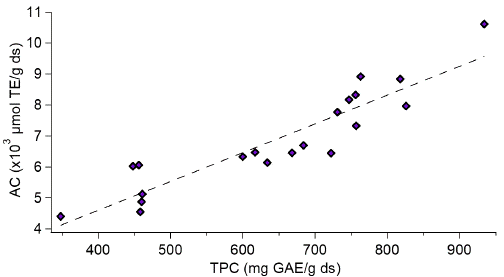
The AC was determined for each mixing point (Table 3), where a linear relationship was observed between the TPC and the AC (Figure 3). This relationship was evaluated by the correlation coefficient (R2), obtaining a value of 0.84. A similar analysis was carried out by Jacobo and Cisneros [34], showing that the antioxidant activity is directly related to the phenolic profiles through a correlation coefficient, obtaining R2 values > 0.68. The values of antioxidant capacity for the mixtures are higher than those quantified for the unpurified extract. The highest AC value is given for the sample containing only dimers (DP value of 2) with optimized values of up to 2.74 times the value of antioxidant capacity concerning the unpurified sample.
The experimental results of AC were adjusted to a complete cubic model, which managed to explain the data obtained in 88.4%, with a p value < 0.05. With the cubic model, the data was adjusted until Eq. (3) was obtained.
Where AC is antioxidant capacity; A: monomer fraction; B: dimer fraction, and C: oligomer fraction. The equation considers the linear effect of each of the variables, A, B, and C with DP values of 1, 2 and > 2, respectively, as well as their interaction and quadratic contribution. It was observed that the order of influence of the variables on antioxidant capacity is B> C> A (Figure 4). On one hand, it can be concluded that B fraction (dimers) is the most influential and will give the greatest antioxidant capacity. On the other hand, AB, AC, BC, and ABC contributions are negative, which suggests that the combination of these fractions hold an antagonistic effect on the antioxidant capacity.
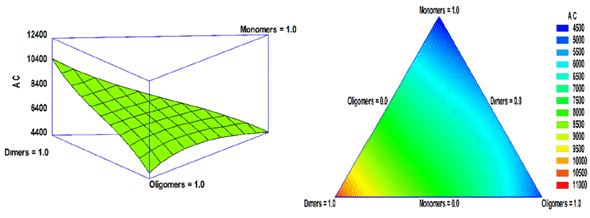
Figure 4 Surface and response contour of the antioxidant capacity with polyphenols of different DP values (monomers, dimers, and oligomers).
According to the results obtained, the maximum antioxidant capacity was achieved for the sample that contained dimers with DP value of 2 (Figure 4). This is because the ability of a molecule to donate its protons and act as free radical scavengers seems to depend primarily on the number of hydroxyl groups and their position on the molecule [35]. Also, dimers have a higher value of antioxidant capacity due to the catechin-type structure with greater oxidability. Here, the reaction generate a significant amount of oxygen radicals in ORAC tests and lead to a higher antioxidant capacity [36,37]. As stated, there is a strong probability that the fraction of oligomers has a greater antioxidant capacity than dimers. However, their antioxidant capacity may be reduced due to the molecular size that creates competitive effects and steric impediments in the generation of oxygen radicals [38,39]. Similarly, the antioxidant potential is related to the planar position in the molecule [14,40]; consequently, a molecule that has these characteristics would be ideal to act as an antioxidant. In contrast, the presence of glycosylation in the molecule can reduce its antioxidant activity [14,41].
As it is shown by Fernández-Pachón et. al., the monomers, corresponding to catechin and epicatechin, have a higher value of antioxidant capacity than dimers when comparing the antioxidant capacity of the standards (monomers, dimers, and trimers) [42]. Although, with processes that involve temperature and pH changes, the concentration of monomers decreases and their antioxidant capacity decrease [43,44]. In this work, a higher antioxidant capacity was obtained for dimers than for monomers. This was probably because dimers have better stability in the extraction and purification process, reflecting a greater antioxidant capacity in the collected extracts.
With the equation developed, it can be suggested that when evaluating each of the polyphenol families, they could provide synergistic effects on the antioxidant capacity. Also, the interaction of two or three families, any combination, brings antagonistic effects on the antioxidant capacity. A pronounced antagonistic effect is observed when monomers are present in the binary or ternary mixture of families with different DP values. This antagonistic effect of the antioxidant capacity can be attributed because the monomers exhibit rapid oxidation in the presence of other oxidative compounds, such as hydroxyl or carbonyl groups in procyanidins and other oligomers [44,45].
Conclusions
The total polyphenolic content for cocoa beans (clone ICS-39) reached 77 mg GAE/g ds, under optimized conditions with an extraction yield of 20.1%. The inhibition of the polyphenol oxidase enzyme contributed to the decrease of polyphenols degradation and raised their antioxidant capacity. This study also confirms that there is an increase in antioxidant capacity each time the total polyphenolic content increases, with a linear correlation coefficient (R2) value of 0.84.
Through the proposed liquid chromatography methodology, it was possible to separate the polyphenols into monomers, dimers, and oligomers (DP values of 1, 2, and > 2, respectively). Dimers showed greater antioxidant capacity, which makes them capable of donate their protons. The behavior of the antioxidant capacity as a function of monomers, dimers, and oligomers was described through a complete cubic model, obtaining a greater antioxidant capacity in dimers and an antagonistic effect when polyphenols of different DP values are mixed.