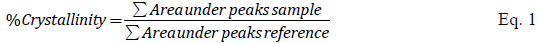Introduction
The ZSM-5 zeolite (Zeolite Socony Mobile 5) is a molecular sieve with a well-defined crystalline structure. It has a three-dimensional framework with micropores that give a high specific surface area. Furthermore, ZSM-5 has an acid site distribution divided between Brönsted and Lewis types [1], [2]. These properties in addition to high thermal stability make ZSM-5 a material with a wide range of applications such as ion exchanger, catalyst, membrane separator, and gas adsorber [3]. Also, it is important to remark that most of these properties are defined by their Si/Al ratio and by its chemical composition [4]. Nevertheless, in catalytic processes, some of the properties have to be partially sacrificed to enhance the others. Accordingly, to avoid extra costs, unnecessary treatments and other similar processes, a well-balanced catalyst is commonly the best alternative.
The conventional ZSM-5 zeolite synthesis method involves a gel formation obtained from different chemical reagents such as sodium silicate, aluminum sulfate, and tetrapropylammonium hydroxide (TPA-OH) [5],[6]. These chemical reagents are expensive, so it is convenient to use lower cost materials such as diatomaceous earth [7]-[11]. Due the importance of the Si/Al ratio in zeolite's properties, there are many studies in relation to modulating the Si/Al ratio of a nonmetallic mineral raw material such as kaolin and diatomaceous earth [7]-[9]. Moreover, there are reports regarding the effect of synthesis parameters on the final Si/Al ratio that indicate that every raw material has a different behavior in relation to the acid pre-treatment and synthesis parameters [12]-[14]. As a result, it is necessary to identify the optimal synthesis conditions for regulating the Si/Al ratio according to the final zeolite application. Thus, it would be a more economical process to treat the diatomaceous earth with mineral acids than to regulate the Si/Al ratio by adding high purity reagents, such as sodium silicate or sodium aluminate. In addition, it is important to consider that Bolivia has natural reserves of diatomaceous earth that are not being used for any application and, to the best of our knowledge, there are no reports regarding the effect of synthesis parameters such as the temperature of the acid pre-treatment on the final properties of the ZSM-5 zeolites [15]. Therefore, in the present research work, we report the effect of the temperature during the acid treatment of Bolivian diatomaceous earth and the synthesis of ZSM-5 zeolites obtained from them. Also, the characterization of properties was carried out for every obtained zeolite, allowing to determine the present crystalline phase, degree of crystallinity, specific surface area, micropore volume and acid site concentration and distribution.
Materials and Methods
Acid pre-treatment of diatomaceous earth
The diatomaceous earth used in the present work was collected from the locality of Bella Vista in the geological formation of Murmuntani in Potosí, Bolivia at 4,067 m.a.s.l. The diatomaceous earth was pre-treated with a 6 M sulfuric acid solution in a proportion of 1:9 (solid: sample solution) in a hydrothermal reactor for 24 h at 4 different temperatures (50, 85, 120 and 150 °C). Subsequently, the hydrothermal reactor was quenched and the product filtered and washed with distillated water until pH was close to 7. The obtained solid was separated and dried overnight at 100 °C.
Synthesis of the ZSM-5 zeolite
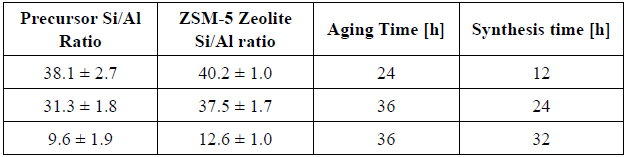
Four zeolites were synthetised from acid pre-treated diatomaceous earth with different Si/Al ratios. The four zeolites were prepared from the same mixture composition: Mixing the acid pre-treated diatomaceous earth with distilled water, sodium hydroxide (Merck pure grade 99%) and TPA-OH (Merck reactive 1M solution) as a structural directional agent (SDA). The molar ratio in the synthesis mixture was: Na2O/SiO2 = 0.18; SiO2/Al2O3 =X; SiO2/TPA-OH = 3.5; H2O/SiO2=30. The synthesis mixture was aged under constant stirring at room temperature and then the pH of the mixture was adjusted to 11 with a 1 M solution of hydrochloric acid. Successive to the aging time, the synthesis mixture was hydrothermally treated in a Teflon stainless steel autoclave at 170 °C. Subsequently, the hydrothermal reactor was quenched and the product was washed with distilled water until the pH reached a value close to 7. Finally, the obtained solid was dried at 100 °C overnight and calcinated at 550 °C for 8 h. The aging time and synthesis time were different for each Si/Al ratio in order to improve the degree of crystallinity of the final zeolite (see Table 1).
NH4+ ion exchange of ZSM-5 zeolite
In order to obtain the protonated form of the zeolite (H-ZSM-5), the ZSM-5 zeolite was mixed with ammonium nitrate 1 M in a reflux system at 80 °C for 12 h. The zeolite to ammonium nitrate ratio was 1:12 (in weight terms). Subsequently, the solid was separated by filtering and washed with distilled water. Finally, the zeolite was dried at 100 °C overnight and calcinated for 8 h at 550 °C. This procedure was carried out twice for each sample.
Characterisation techniques
The Si/Al ratio of the acid pre-treated diatomaceous earth and the H-ZSM-5 zeolite was quantified by the X-ray fluorescence (XRF) technique. The readings were carried out with a Thermo Scientific Niton XL3t Ultra spectrometer. The Si/Al ratio was quantified using a calibration curve developed from samples prepared with different concentrations of pure silicon oxide and pure alumina. The statistical evaluation (C.I. 95%) was undertaken using the "IBM SPSS statistics 22" software.
The identification of crystalline phases and the ascertainment of the degree of crystallinity of the samples were determined by means of the X-ray diffraction (XRD) technique. The readings were carried out on a PANalytical X-ray Diffractometer with an X-ray copper tube (Kα= 1.54 Å) and an Xcelerator detector. The operational conditions applied to the X-ray tube were 40 mA and 40 kV. The analysis range was from 5 to 60 °, 2θ Bragg degrees. The analyses were carried out in triplicate.
The degree of crystallinity (Eq. 1) [7] was assessed by calculating the area under the principal peaks in the 2θ region between 22.0 to 25.0 ° and comparing it with a high crystalline synthetic zeolite.
The identification and quantification of the acid sites were carried out by the Ammonia Temperature Programmed Desorption (NH3-TPD) technique. The readings were carried out on a ChemBET TPR/TPD Quantachrom Analysis equipment. A known weight for zeolite was placed in the analysis cell. Then the zeolite was degassed at 250 °C for 30 min and activated at 550 °C for 1 h under a flow of He (15 mL/min). Subsequently, a flow of 15 mL/min (5% NH3/He) was passed through the cell and ammonia adsorption was carried out for 1 h at 100 °C. Finally, the gas flow was changed to He (15 mL/min) and heated to 850 °C at 9 °C/min. The signals were registered with a TCD detector. For NH quantification previous analysis were carried out to determine a calibration factor between the signal area detected by the TCD and the previous known NH3 volume. Due the destructive behaviour of the technique, the analysis was carried out only once per sample.
Nitrogen Physisorption was carried out on a ChemBET TPR/TPD Quantachrom Analysis equipment. The pre-treatment of the samples was the same as for the NH3-TPD analysis. The weighted sample was placed in the analysis cell and different proportions of N2/He (P/P0= 0.10 - 0.35) was used according to the BET method. (The analyses were carried out in triplicate).
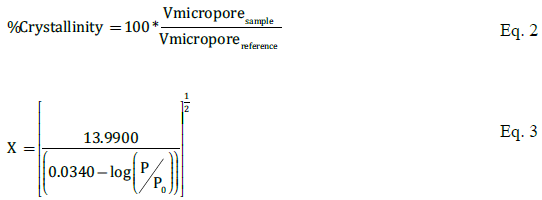
To quantify the specific surface area, the BET method was applied with the sorption data. The T-Plot method was applied to quantify the micropore volume, micropore area, external area and degree of crystallinity (Eq. 2). The T-Plot method is applied in samples that present microporosity. To estimate the thickness of the pore we used the zeolite equation (Eq. 3) as the literature suggests [16].
Finally, the morphology and particle size were performed by an external laboratory as an analysis service.
Results and Discussion
Diatomaceous earth pre-treatment: The effect of temperature on the Si/Al ratio
It is well known that diatomaceous earth is often pre-treated with mineral acid in order to reduce the concentration of undesired metallic species (e.g., alkali metals) and regulate the Si/Al ratio [13], [17]. To achieve this, the diatomaceous earth was mixed up with 6M sulfuric acid at five different temperatures for 24 h. The applied temperature during the acid pre-treatment caused significant changes in the Si/Al ratio (see Figure 1) in comparison to the non-treated diatomaceous earth (Si/Al=6.18).
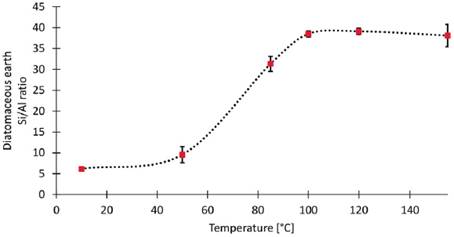
Figure 1 Effect of the pre-treatment temperature during the acid pre-treatment of diatomaceous earth on the Si/Al ratio.
The Si/Al ratio of diatomaceous earth can be modulated from Si/Al=6.2 (natural raw material) up to Si/Al=38.1 by controlling the acid pre-treatment temperature (See Supplementary information section). The highest Si/Al ratio was achieved at 120 °C. At higher temperatures no significant changes were observed in the Si/Al ratio. At lower temperatures the Si/Al ratio decreases because aluminum solubility increases at higher temperatures [13], [17].
Synthesis of ZSM-5 zeolite from acid pre-treated diatomaceous earth
The acid pre-treated diatomaceous earth samples with different Si/Al ratios were used as precursors to obtain ZSM-5 zeolite. The diffractograms shown in Figure 2 indicate that only the precursor with Si/Al=38.1 (highest ratio) has achieved a ZSM-5 zeolite structure after synthesis conditions. The remainder of the diatomaceous earth precursors do not present any clear crystalline structure after synthesis. The latter may be attributed to the different contents of aluminum in each precursor, since the greater aluminum content reduces the rate of crystal growth because aluminum intrusion is a disruptive process that requires more energy to develop [18].
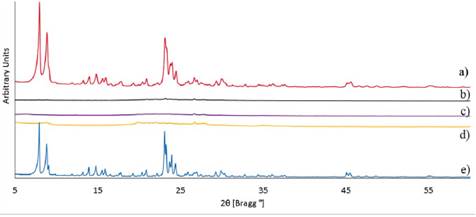
Figure 2 XRD diffractograms of zeolite synthesis from diatomaceous earth precursors: a) Si/Al=38.1, b) Si/Al=31.3, c) Si/Al=9.6, d) pure diatomaceous earth (Si/Al=6.2) and e) reference ZSM-5 zeolite obtained from pure reagents (Si/Al=43.8).
The low intensity and poor definition of the signals observed in Figure 2, suggest that it is necessary to improve aging and synthesis conditions. During aging time, ZSM-5 nuclei are formed and the process is affected by the presence of aluminum. At this point, aluminum interacts with [OH-] anions reducing the interaction between silica and [OH-]. These interactions lead to delay the Si-O-Si nuclei formation [18], [19]. On the other hand, the synthesis time is also affected by the presence of aluminum. Due to the disruptive nature of aluminum, crystal development requires more energy. As a consequence, more aluminum content will cause crystal growth to proceed at a slower rate [18] - [20]. So, to improve the degree of crystallisation of the ZSM-5 zeolite the aging time and the synthesis time were modified for each acid pre-treated diatomaceous earth sample.
Optimisation of ZSM-5 zeolite synthesis based on diatomaceous earth with different Si/Al ratios
After improving the "aging and synthesis time" (see Table 2), a typical ZSM-5 zeolite diffractogram was introduced from all the acid pre-treated diatomaceous earth samples, as can be seen in Figure 3.
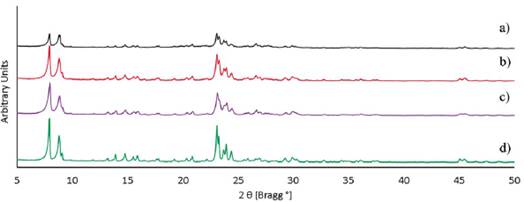
Figure 3 XRD diffractograms after improving the aging and synthesis time of ZSM-5 zeolite using: a) Si/Al=9.6 b) Si/Al=31.3 c) Si/Al=40.2 and d) pure reagents as a reference Si/Al=43.8.
A direct ratio can be noted between the aluminum content and the degree of crystallinity in the final ZMS-5, in accord with Jacobs et al. [21], i.e., the lower the amount of aluminum species the higher degree of crystallinity (see Table 3). Thus, ZSM-5 zeolites were obtained from acid pre-treated diatomaceous earth samples with different Si/Al ratios. The zeolite with the lowest ratio (Si/Al=12.6) was obtained with a 60% of amorphous phase and a 40.0% of degree of crystallinity, being a predominantly amorphous material. And the ZSM-5 with the highest ratio (Si/Al=40.2) was obtained with a 96.6% degree of crystallinity and 3.4% of amorphous phase, being a predominantly crystalline material. The synthesis of the ZSM-5 zeolites with different crystallinity and amorphous material percentages was assessed by modulating the "aging time and synthesis time".
Table 2 Average Si/Al ratio obtained from diatomaceous earth after acid treatment at different temperatures.
| Temperature [°C] | Si/Al ratio |
|---|---|
| 155 | 38.1±2.7 |
| 120 | 39.1±0.8 |
| 100 | 38.5±0.7 |
| 85 | 31.3±1.8 |
| 50 | 9.6±1.9 |
| No treatment | 6.2±0.3 |
Table 3 Summary synthesis of the obtained ZSM-5 zeolites from acid pre-treated diatomaceous earth precursors.
| Time [h] | Diatomaceous Earth Si/Al ratio | Final ZSM-5 zeolite | ||
|---|---|---|---|---|
| Aging | Synthesis | Precursors Si/Al Ratio | ZSM-5 Zeolites Si/Al Ratio | Degree of Crystallinity [%] |
| 72 | 120 | 43.8 (Reference) | 43.8 | 100.0 ± 8.6 |
| 24 | 12 | 38.1 ± 2.7 | 40.2 ± 1.0 | 96.6 ± 10.2 |
| 72 | 24 | 31.3 ± 1.8 | 37.5 ± 1.7 | 83.7 ± 7.8 |
| 72 | 32 | 9.6 ± 1.9 | 12.6 ± 1.0 | 45.0 ± 3.4 |
Nitrogen Physisorption
Sorption Isotherm
The obtained zeolites have different nitrogen adsorption capacities. A direct correlation can be appreciated between the adsorbed volume and the Si/Al ratio: A high Si/Al ratio leads to an increase in the adsorption capacity of the zeolite (See Table 4). This behaviour can be explained by pore collapse due to the intrusion of aluminum in the crystalline structure. A high aluminum content leads to structure instability causing the structure to collapse within itself [18]-[20].
Table 4 Nitrogen adsorption from sorption experiment for ZSM-5 zeolites with different Si/Al ratios.
| ZSM-5 Zeolite Si/Al ratio → | 43.8 | 40.2 | 37.5 | 12.6 |
|---|---|---|---|---|
| Relative Pressure | Adsorbed Vol. [cm3/g] | Adsorbed Vol. [cm3/g] | Adsorbed Vol. [cm3/g] | Adsorbed Vol. [cm3/g] |
| 0.10 | 105.7 | 124.6 | 87.7 | 47.1 |
| 0.15 | 107.9 | 118.9 | 112.9 | 55.2 |
| 0.20 | 110.6 | 127.7 | 102.5 | 55.7 |
| 0.25 | 112.1 | 124.5 | 108.1 | 45.6 |
| 0.30 | 114.6 | 126.2 | 98.9 | 71.5 |
| 0.35 | 114.1 | 128.3 | 95.8 | 58.0 |
Bruner Emmet Teller (BET)
Based on Table 5 it can be observed that the specific surface area is dependent on the Si/Al ratio: Low aluminum content leads to an increase in the specific surface area and, high aluminum content has the opposite effect in concordance with the literature [21]. This behaviour can be explained by the disruptive effect of the aluminum on the ZSM-5 crystalline structure. A high aluminum content leads to a reduction of the interaction between SiO2 species with [OH-] and, in consequence the degree of crystallinity decreases gradually. Accordingly, the specific surface area also decreases [18].
Table 5 BET specific surface area, determination of coefficient and CBET constant values.
| ZSM-5 zeolite Si/ Al ratio | Specific Surface Area by BET [m2/g] | Determination of Coefficient in the BET range (P/ P0=0.1-0.35) | CBET Constant |
|---|---|---|---|
| 43.8 (Reference) | 290.0 ± 17.9 | 0.99 | -29.8 |
| 40.2 ± 1.0 | 300.0 ± 14.8 | 0.99 | -29.0 |
| 37.5 ± 1.7 | 235.1 ± 29.2 | 0.96 | -20.6 |
| 12.6 ± 1.0 | 229.0 ± 23.7 | 0.99 | 38.0 |
T-Plot method
On Table 6, it can be appreciated that the micropore properties (volume and internal surface area) and the external surface area are related to the Si/Al ratio as were the BET results. The micropore volume is related to the crystallinity of the zeolite: If the crystalline structure of the zeolite is conformed in a perfect system, the micropore volume reaches a maximum value, because in a perfect system there is no loss in the volume of the pores. In our case, the micropore volume of the reference zeolite is 0.135 cm3/g in concordance with the reports of Sayari et al. and Solcová et al. [22]. Furthermore, at high Si/Al ratios (38.1) the micropore volume is almost constant, while at low Si/Al ratios (9.6) it considerable reduces. This behaviour is a consequence of loses of crystallinity between Si/Al= 38.1 and Si/Al=9.6 [18]. Because the internal surface area and the micropore area are directly related to the micropore volume these results present the same behaviour in relation to the Si/Al ratio. Both cases have the same explanation. The intrusion of aluminum species in the synthesis mixture leads to a decrease in crystallinity, reducing the micropore properties including the micropore volume and surface areas.
Table 6 T-plot method results of the obtained zeolites.
| ZSM-5 Zeolite Si/Al Ratio | Micropore Volume [cm3/g] | Micropore Area [m2/g] | External Surface Area [m2/g] | % of Crystallinity | Determination of Coefficient |
|---|---|---|---|---|---|
| 43.8 | 0.14 | 268 | 21 | 100.0 | 0.96 |
| 38.1 ± 1.0 | 0.14 | 276 | 23 | 100.0 | 0.98 |
| 31.3 ± 1.7 | 0.08 | 190 | 44 | 58.5 | 0.52 |
| 9.6 ± 1.0 | 0.05 | 206 | 22 | 35.0 | 0.65 |
Scanning Electron Microscopy (SEM)
Particle Size and Morphology
Particle size and morphology were studied by way of SEM as can be appreciated in figure 4. The calibration bar presented in the lower part of figure 4, was used to measure the average particle size for each zeolite. The summary of the particle sizes is shown on Table 7.
Table 7 Average particle size for each ZSM-5 zeolite synthetised from diatomaceous earth.
| ZSM-5 Zeolite Si/Al ratio | Average Particle Size [um] |
|---|---|
| 40.2 | 2.1 ± 0.3 |
| 37.5 | 2.1 ± 0.4 |
| 12.6 | 2.5 ± 0.4 |
Typical rounded shape crystals were obtained from the synthesis of ZSM-5 zeolites from diatomite (figure 5 A, B and C). This shape was expected according to references from researchers who also worked with diatomaceous earth under similar conditions [7]. Also, in figure 4 D it can be appreciated that the round particles are formed by the union of different orthorhombic crystals that develop as twin crystals side by side with a single crystal shape similar to the results found by Sanhueza et al [9]. It is possible to observe laminar residues of diatomaceous earth (shown in figure 4 B and C inside the circles) mainly in the samples with a lower Si/Al ratio. These residues evidence that not all the treated diatomite was transformed into ZSM-5 zeolite.
Ammonia temperature programmed desorption (NH 3 -TPD)
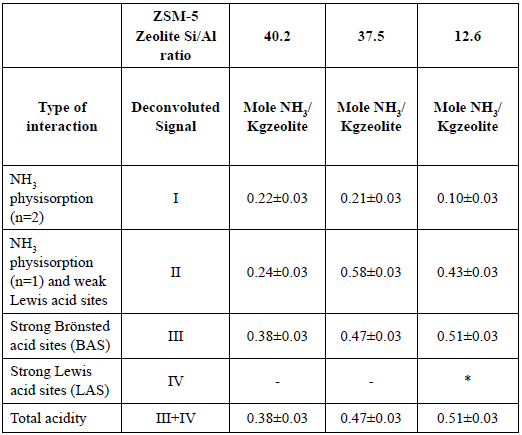
Figure 5 shows the experimental NH3-TPD profiles for each zeolite. The profiles have a typical form of ZSM-5 zeolite from NH3-TPD-experiments [23], [24]. Four deconvoluted signals were assigned from the NH3-TPD profile for the ZSM-5 zeolite with Si/Al=12.6. For zeolites with Si/Al=40.2 and Si/Al=37.5 only three deconvoluted signals were observed. The first deconvoluted signal has a maximum temperature between 140 to 200 °C. The second deconvoluted signal has a maximum temperature between 220 to 370 °C. The third deconvoluted signal has a maximum temperature between 600 to 750 °C. And the fourth signal has a maximum temperature around 850 °C. The first signal can be attributed to physically desorbed NH3, where the NH3 comes from NH4 +nNH3 species adsorbed on the zeolite surface [23], [25]. The second signal is partially attributed to physically desorbed NH3 and to interactions between NH3 and weak Lewis acid sites [23], [26]. Lewis acid sites are generated by the presence of cations in the crystalline structure for charge compensation [26], [27]. The third signal is due to strong Brönsted acid sites (BAS) [23]. Finally, the fourth signal is due to strong Lewis acid sites (LAS) [28]. LAS are generated by the presence of extra framework aluminum species (EFAL).
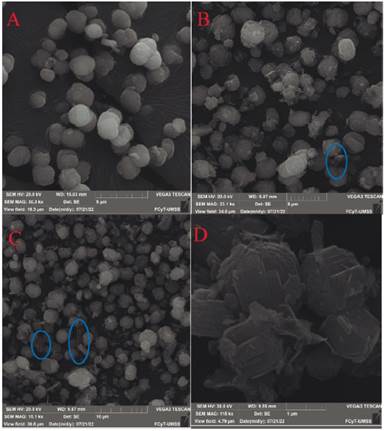
Figure. 4 SEM micrographs of ZSM-5 zeolites with: A) Si/Al=40.2 B) Si/Al=37.5 C) Si/Al=12.6 and D) Additional zoom on the surface of ZSM-5 with Si/Al=40.2.
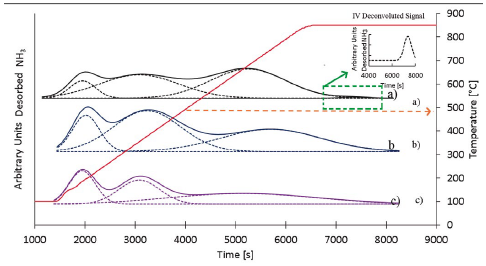
Figure 5 NH3- TPD profiles for: a) ZSM-5 with Si/Al=12.6 b) ZSM-5 with Si/ Al=37.5 and c) ZSM-5 with Si/Al =40.2.
Table 8 presents the behaviour of the different acid sites in relation to the Si/Al ratio of the ZSM-5 zeolites. Weak Lewis acid sites are dependent on the acid pre-treatment temperature since high temperatures permit extracting more interfering species, such as metallic species [25], [29] so less weak Lewis acid sites are expected. This is evident with the acid pre-treated diatomaceous earth at 120 °C (Si/Al=40.2) which reduces the quantity of Lewis acid sites by half as compared to the acid pre-treated diatomaceous earth treated at 85 °C (Si/Al=37.5), see Table 8.
The BAS are related to the content of aluminum in the structure of the zeolite. The more aluminum the structure has, the more it needs cations to compensate the generated negative charge. These cations could come from undesired species, such as metallic species (weak Lewis acid sites) or by way of ion exchange. The studied zeolites were in a protonated form (H-ZSM-5), so the final aluminum concentration will determinate the BAS concentration [23].
The LAS are related to the content of EFAL in the zeolite. At elevated aluminum content EFAL are formed during the synthesis process due the disruptive nature of aluminum intrusion in the crystalline structure. In the zeolite with Si/Al=12.6 the concentration of aluminum is too high, so part of the Al forms EFAL because the ZSM-5 zeolite undergoes a dealumination process in order to stabilise itself [30]. As a consequence, the aluminum forms penta-coordinated species (EFAL) and remains as part of the aggregate [31]. This phenomenon explains the existence of the fourth signal in the ZSM-5 zeolite Si/Al=12.63 ratio [18], [23], [31].
Conclusions
It is possible to modulate the Si/Al ratio of natural Bolivian diatomaceous earth by changing the temperature of the acid pre-treatment. The pre-treated diatomaceous earth was used to synthetise three ZSM-5 zeolites with different Si/Al ratios in the range 12.6 - 40.2. The obtained ZSM-5 zeolites have different physicochemical properties. The degree of crystallisation, micropore volume, internal surface area and external surface area are dependent on the Si/Al ratio. The results show that high aluminum content leads to a loss of crystallinity, possibly due to pore collapse. The concentration and distribution of acid sites (BAS and LAS) are dependent on the zeolite's Si/Al ratio. High total acidity amounts (>0.52 molNH3/ Kgzeolite) are found at low Si/Al ratios and are composed of strong Brönsted and strong Lewis acid sites. At high Si/Al ratios a reduction of the total acidity amount is reported (<0.38 molNH3/Kgzeolite). Further investigation is needed to develop the potential applications of the obtained zeolites in areas such as heterogeneous catalysis, gas adsorption, etcetera.