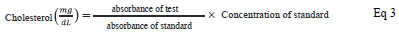Introduction
Diabetes mellitus is a spectrum of metabolic disorders resulting from myriad pathogenic mechanisms, all of which lead to hyperglycemia [1]. In recent years, diabetes has become one of the leading causes of death worldwide. In 2016, the World Health Organisation reported around 1.6 million deaths globally due to diabetes. The number of diabetes cases worldwide in 2019 was 463 million and by 2045 it could increase to 629 million. In India, 77 million suffer from diabetes and could be further increased and India has the second highest number of cases in the world [2]. Oxidative stress plays an important role in the pathogenesis of typical long-term diabetic complications such as neuropathy and microangiopathy. Further, dynamic modification of intracellular redox sensors by way of reactive oxygen species in addition to other post-translational modifications such as protein phosphorylation, acetylation or ubiquitination also play a role in the development of the diabetes [3]. Thiazolidinone is one of the important and fundamental central structures for the synthesis of biologically active compounds [4]. Thiazolidin-4-one is a well-known scaffold for producing the desired biological activities: Antidiabetic [5], antioxidant [6], anticancer [7], antitubercular [8], antimicrobial [9], anticonvulsant [10], analgesic and anti-inflammatory [11] and antihyperlipidemic [12].
The abovementioned facts triggered an interest in taking up this investigation, wherein molecular architecture having a 2,3-disubstituted 4-thiazolidinone core derived from sulfanilamide was designed with an anticipation of good antidiabetic activity. Spectral characterisation, docking studies with peroxisome proliferative activated receptor-y (PPAR-y), and in silico ADME parameter studies were also undertaken.
Materials and Methods
Chemicals employed were of laboratory reagent grade and used as received. Sulphanilamide, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), and p-hydroxybenzaldehyde were purchased from Loba Chemie Pvt Limited, Mumbai, India. The other substituted aldehydes, 1,4-dioxane, thioglycolic acid, anhy-drous zinc chloride, ascorbic acid, and D-fructose were procured from SD Fine Chemicals, Mumbai, India. p-Tolualdehyde was purchased from Sigma Aldrich, Steinheim, USA. p-Fluorobenzaldehyde was obtained from Spectrochem, Mumbai, India; and 2,4-dichlorobenzaldehyde, 2,6-dichlo-robenzaldehyde, and p-bromobenzaldehyde from HiMedia Laboratories Pvt. Ltd. Mumbai, India. The glucose, cholesterol, and triglyceride kits were purchased from Erba Mannheim, Sikkim, India. Pioglitazone hydrochloride was provided by USV Private Limited, Solan, India. Thin layer chromatography using pre-coated aluminium sheets with silica gel 60 F254 (Merck, Darmstadt, Germany) was performed using a combination of sol-vents in different ratios as mobile phase. Spots were visualised by iodine vapor and/or UV light. Yields refer to crude products.
Melting points were determined on an open capillary apparatus (Veego VMP-DS Mumbai, India) and are uncorrected. The compounds were characterised by ultraviolet (UV) spectra (UV 1601 Shimadzu, Kyoto, Japan) and infrared (IR) spectra as KBr pellets (Shimadzu and Thermo Scientific Nicolet iS5, Japan). Proton nuclear magnetic resonance (1H NMR) was recorded by means of DMSO-d6 (Bruker Avance Neo 500 MHz, Germany) while 13C NMR was recorded at 125 MHz. Mass spectra (MS) were registered by chemical ionisation technique (Waters-SynaptG2, Massachusetts, USA).
Animals
Sprague Dawley rats of either sex weighing between 250 and 350 g and Swiss albino female mice weighing from 25 to 40 g were obtained from the central animal house of the HSK College of Pharmacy, Bagalkote. The animals were housed under standard conditions (temperature 25 ±1°C, relative humidity (50-55%)) for 12 h dark and light cycle. They were given standard laboratory feed (Pranava Agro Industries Ltd., Sangli, India) and water ad libitum and acclimatised for 7 days before experimentation. Food was withdrawn 18 h before the experiment, but free access to water was allowed. The experiments were performed on randomly formed groups during the light phase of the cycle and the animals were used for one experiment only. The test compounds and standard drug were administered p.o. as aqueous suspension in 0.5% Tween-80. The animals in the vehicle control group received 0.5% aqueous Tween-80. Blood samples were collected from retro orbital flexus under ether anaesthesia. The study was approved and conducted under the guidelines of institutional animal ethics committee constituted as per committee for the control and supervision of experiments on animals, Ministry of Environment and Forestry, Government of India (F.No. HSK College of Pharmacy Bagalkot/IAEC//HSKCOP/AUG2021/PG13).
Synthesis of Schiff bases 3a-n
Sulfanilamide (40 mmol) 1 and substituted aldehydes (40 mmol) 2a-n were dissolved in 20 mL ethanol and the reaction mixture was refluxed for 4-5 h. Completion of reaction was monitored by TLC (n-hexane: ethyl acetate 1:2). The excess of ethanol was distilled, and the product 3a-n was settled after several hours and was filtered and washed with cold ethanol multiple times [13].
4-(benzylideneamino)benzenesulfonamide 3a
White crystals; Yield: 38%; MP: 192-194 oC; Rf: 0.77; IR (KBr, cm-1): 3296.35 (NH str), 3012.81 (Ar-CH str), 1624 (C = N str), 1575 (Ar-C = C str), 1334 (SO2 str).
4-((4-methylbenzylidene)amino)benzenesulfonamide 3b
White crystals; Yield: 43.93%; MP: 198-200 oC; Rf: 0.78; IR (KBr, cm-1): 3277 (NH str), 2993 (Ar-CH str), 1622 (C = N str), 1581 (Ar-C = C str), 1330 (SO2 str).
4-((4-methoxybenzylidene)amino)benzenesulfonamide 3c
White crystals; Yield: 46.11%; MP: 200 oC; Rf: 0.69; IR (KBr, cm-1): 3277 (NH str), 2954 (Ar-CH str), 1610 (C = N str), 1577 (Ar-C = C str), 1322 (SO2 str).
4-((2-hydroxybenzylidene)amino)benzenesulfonamide 3d
Yellowish orange crystals; Yield: 45.0%; MP: 218 oC; Rf: 0.76; IR (KBr, cm-1): 3342 (OH str), 3230 (NH str), 3012.81 (Ar-CH str), 1618 (C = N str), 1562 (Ar-C = C str), 1301 (SO2 str).
4-((4-hydroxybenzylidene)amino)benzenesulfonamide 3e
Light yellowish powder; Yield: 69.05%; MP: 212 oC; Rf: 0.67; IR (KBr, cm-1): 3346 (OH str), 3266 (NH str), 3011.66 (Ar-CH str), 1620 (C = N str), 1576 (Ar-C = C str), 1326 (SO2 str).
4-((4-(dimethylamino)benzylidene)amino)benzenesulfonamide 3f
Yellowish powder; Yield: 6.82%; MP: 220 oC; Rf: 0.83; IR (KBr, cm-1): 3277.06 (NH str), 2900 (Ar-CH str), 1604.77 (C = N str), 1571 (Ar-C = C str), 1328 (SO2 str).
4-((3,4-dimethoxybenzylidene)amino)benzenesulfonamide 3g
White powder; Yield: 57.98%; MP: 200-202 oC; Rf: 0.55; IR (KBr, cm-1): 3277 (NH str), 2954 (Ar-CH str), 1608 (C = N str), 1568 (Ar-C = C str), 1330 (SO2 str), 1278 (Ar-C-O-C str).
4-((4-chlorobenzylidene)amino)benzenesulfonamide 3h
White crystals; Yield: 86.16%; MP: 206 oC; Rf: 0.75; IR (KBr cm-1): 3320 (NH str), 3010 (Ar-CH str), 1615 (C = N str), 1566 (Ar-C = C str), 1296 (SO2 str), 846 (C-Cl str).
4-((2,4-dichlorobenzylidene)amino)benzenesulfonamide 3i
Light yellowish crystals; Yield: 44.93%; MP: 210 oC; Rf: 0.80; IR (KBr, cm-1): 3348 (NH str), 3050 (Ar-CH str), 1618 (C = N str), 1565 (Ar-C = C str), 1298 (SO2 str), 831 (C-Cl str).
4-((2,6-dichlorobenzylidene)amino)benzenesulfonamide 3j
White powder; Yield: 34.55%; MP: 178 oC; Rf: 0.83; IR (KBr, cm-1): 3347 (NH str), 3056 (Ar-CH str), 1619 (C = N str), 1568 (Ar-C = C str), 1299 (SO2 str), 833 (C-Cl str).
4-((4-bromobenzylidene)amino)benzenesulfonamide 3k
White crystals; Yield: 98.3%; MP: 222 oC; Rf: 0.75; IR (KBr, cm-1): 3290 (NH str), 2987 (Ar-CH str), 1622 (C = N str), 1577 (Ar-C = C str), 1330 (SO2 str), 840 (C-Br str).
4-((4-nitrobenzylidene)amino)benzenesulfonamide 3l
Yellowish orange powder; Yield: 18.83%; MP: 178-180 oC; Rf: 0.85; IR (KBr, cm-1): 3263 (NH str), 3091 (Ar-CH str), 1627 (C = N str), 1591 (Ar-C = C str), 1340 (SO2 str), 1294 (C-NO2).
Synthesis of 2,3-disubstitued thiazolidin-4-ones (4a-n)
The substituted Schiff bases 3a-n (10 mmol), thioglycolic acid (20 mmol), and anhydrous zinc chloride (0.2 g) were added to10 mL 1,4-dioxane and the mixture was refluxed for 20 h. The progress of the reaction was monitored by TLC (n-hexane: ethyl acetate 3:2). The reaction mixture was allowed to cool and poured into crushed ice. The crude solid of 2,3-disubstituted thiazolidin-4-ones 4a-n obtained was filtered, washed thoroughly with sodium bicarbonate followed by ice cold brine solution. The compounds were recrystallised with aqueous alcohol (30:70) [14].
4-(4-oxo-2-phenylthiazolidin-3-yl)benzenesulfonamide 4a
Orange powder, Yield: 28.74%; MP: 122 oC; Rf: 0.33; IR (KBr, cm-1) : 3422.20 (NH str), 1630.63 (C = O str), 1384.70 (SO2 str), 618 (C-S str), H NMR (500 MHz, δ, ppm): 4.10 (q, 2H, 5-CH2, Thiaz) 6.39 (s, 1H, 2-CH, Thiaz) 7.03 (s, 2H, SO2NH2) 7.10 (m, 1H, 4" ArH) 7.21 (m, 2H, 3" & 5" ArH) 7.35 (m, 2H, 2" & 6" ArH) 7.42 (d, 2H, 2' & 6' ArH) 7.76 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 32.53 (1C, 5C, Thiaz) 62.64 (1C, 2C, Thiaz) 121.5 (2C, 2' & 6' ArC) 125.8 (2C, 2" and 6" ArC) 126.2 (1C, 4" ArC) 128.4 (2C, 3" and 5" ArC) 129.3 (2C, 3' & 5' ArC) 135.4 (1C, 4' ArC) 140.6 (1C, 1" ArC) 143.8 (1C, 1' ArC) 170.40 (1C, C = O); Mass (m/z): 335.01 (M+ +1), 336.01 (M++2).
4- (4-oxo-2-(p-tolyl)thiazolidin-3-yl)benzenesulfonamide 4b
Orange powder, Yield: 31%; MP: 182-184 oC; Rf: 0.37; IR (KBr, cm-1) : 3406.18 (NH str), 2850 (Ali CH str), 1650 (C = O str), 1384.71 (SO2 str), 618.52 (C-S str), 1H NMR (500 MHz, δ, ppm): 2.39 (s, 3H, CH3) 4.4 (q, 2H, 5- CH2,Thiaz) 5.7 (s, 1H, 2-CH, Thiaz) 6.86 (s, 2H, SO2NH2) 7.34 (m, 2H, 3" & 5" ArH) 7.38 (m, 2H, 2" & 6" ArH) 7.5 (m, 2H, 2' & 6' ArH) 7.8 (m, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 20.4 (1C, CH3) 32.76 (1C, 5C, Thiaz) 65.68 (1C, 2C, Thiaz) 121.6 (2C, 2' & 6' ArC) 127.9 (2C, 2" and 6" ArC) 128.4 (2C, 3" and 5" ArC) 129.5 (2C, 3' & 5' ArC) 136.4 (1C, 4' ArC) 137.1 (1C, 1" ArC) 137.6 (1C, 4" ArC) 144.8 (1C, 1' ArC) 169.38 (1C, C = O); Mass (m/z): 349.07 (M++1).
4-(2-(4-methoxyphenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4c
Orange powder, Yield: 23.07%; MP: 220 oC; Rf: 0.77; IR (KBr, cm-1) : 3418.04 (NH str), 2852 (Ali CH str), 1655 (C = O str), 1384.86 (SO2 str), 1333.29 (Ar-C-O-C str), 618.33 (C-S str), 1H NMR (500 MHz, δ, ppm): 3.76 (s, 3H, OCH3) 4.4 (q, 2H, 5-CH2,Thiaz) 6.36 (s, 1H, 2-CH, Thiaz) 6.71 (m, 2H, SO2NH2) 6.72 (m, 2H, 3" & 5" ArH) 7.40 (d, 2H, 2' & 6' ArH) 7.72 (d, 2H, 3' & 5' ArH) 7.82 (d, 2H, 2" & 6" ArH); 13C NMR (125 MHz, δ, ppm): 33.76 (1C, 5C, Thiaz) 55.8 (1C, OCH3) 70.53 (1C, 2C, Thiaz) 113.8 (2C, 3" and 5" ArC) 120.2 (2C, 2' & 6' ArC) 128.3 (2C, 3' & 5' ArC) 129.9 (2C, 2" and 6" ArC) 130.3 (1C, 1" ArC) 135.4 (1C, 4' ArC) 144.8 (1C, 1' ArC) 157.6 (1C, 4" ArC) 170.38 (1C, C = O); Mass (m/z): 365.02 (M++1), 366 (M++2).
4-(2-(2-hydroxyphenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4d
Orange powder, Yield: 47.14%; MP: 202-204 oC; Rf: 0.7; IR (KBr, cm-1) : 3345.60 (OH str), 3248.17 (NH str), 2923 (Ali CH str) 1618.84 (C = O str), 1384.77 (SO2 str), 618.57 (C-S str), 1H NMR (500 MHz, δ, ppm): 4.4 (q, 2H, 5-CH2 Thiaz) 5.7 (s, 1H, 2-CH, Thiaz) 6.99 (m, 2H, 3" & 5" ArH) 7.0 (m, 1H, 4" ArH) 7.4 (m, 1H, 6" ArH ) 7.39 (s, 2H, SO2NH2) 7.56 (d, 2H, 2' & 6' ArH) 7.8-7.9 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 32.6 (1C, 5C, Thiaz) 65.3 (1C, 2C, Thiaz) 115.8 (1C, 5" ArC) 119.2 (1C, 1" ArC) 121.9 (1C, 3" ArC) 122.2 (2C, 2' & 6' ArC) 127.8 (1C, 2" ArC) 128.3 (1C, 4" ArC) 129.4 (2C, 3' & 5' ArC) 136.4 (1C, 4' ArC) 145.8 (1C, 1' ArC) 152.9 (1C, 6" ArC) 170.6 (1C, C = O); Mass (m/z): 351.05 (M++1).
4-(2-(4-hydroxyphenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4e
Brownish powder, Yield: 2.5%; MP: 230 oC; Rf: 0.28; IR (KBr, cm-1) : 3358.68 (OH str), 3253 (NH str), 1650 (C = O str), 1384.23 (SO2 str), 618.34 (C-S str), 1H NMR (500 MHz, δ, ppm): 3.98 (q, 2H, 5-CH2,Thiaz) 6.46 (s, 1H, 2-CH, Thiaz) 6.69 (d, 2H, 3" & 5" ArH) 6.88 (s, 2H, SO2NH2) 7.46 (d, 2H, 2' & 6' ArH) 7.62 (d, 2H, 2" & 6" ArH) 7.73 (d, 2H, 3' & 5' ArH) 9.02 (s, 1H, OH); 13C NMR (125 MHz, δ, ppm): 31.5 (1C, 5C, Thiaz) 70.6 (1C, 2C, Thiaz) 114.8 (2C, 3" and 5" ArC) 121.2 (2C, 2' & 6' ArC) 129.3 (2C, 3' & 5' ArC) 131.1 (2C, 2" and 6" ArC) 131.8 (1C, 1" ArC) 136.3 (1C, 4' ArC) 143.8 (1C, 1' ArC) 156.6 (1C, 4" ArC) 168.38 (1C, C = O); Mass (m/z): 351.04 (M++1).
4-(2-(4-(dimethylamino)phenyl)-4-oxothiazolidin-3-yl) benzenesulfonamide 4f
Dark brownish, Yield:19.09%; MP: 158 oC; Rf: 0.57; IR (KBr, cm-1) : 3424.72 (NH str), 2870 (Ali CH str), 1606 (C = O str), 1384.46 (SO2 str), 618.21 (C-S str), 2H NMR (500 MHz, δ, ppm): 3.01 (s, 6H, N(CH3)2) 3.92 (q, 2H, 5-CH2,Thiaz) 6.38 (s, 1H, 2-CH, Thiaz) 6.70 (d, 2H, 3" & 5" ArH) 6.82 (s, 2H, SO2NH2) 7.08 (d, 2H, 2" & 6" ArH) 7.40 (d, 2H, 2' & 6' ArH) 7.74 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 32.6 (1C, 5C, Thiaz) 41.6 (2C, N(CH3)2) 70.8 (1C, 2C, Thiaz) 112.8 (2C, 3" and 5" ArC) 122.5 (2C, 2' & 6' ArC) 126.4 (2C, 2" and 6" ArC) 127.9 (1C, 1" ArC) 128.6 (2C, 3' & 5' ArC) 136.8 (1C, 4' ArC) 143.9 (1C, 1' ArC) 148.2 (1C, 4" ArC) 170.42 (1C, C = O); Mass (m/z): 378.01 (M++1).
4-(2-(3,4-dimethoxyphenyl)-4-oxothiazolidin-3-yl) benzenesulfonamide 4g
Dark brownish, Yield: 1.26%; MP: 90 oC; Rf: 0.46; IR (KBr, cm-1) 3370.80 (NH str), 2924.94 (Ali CH str), 1674.77 (C = O str), 1384.68 (SO2 str), 1333.29 (Ar-C-O-C str), 618.80 (C-S str), 2H NMR (500 MHz, δ, ppm): 3.70 (s, 3H, OCH3) 3.81 (s, 3H, OCH3) 4.02 (q, 2H, 5-CH2,Thiaz) 6.46 (s, 1H, 2-CH, Thiaz) 6.82 (m, 1H, 3" ArH) 6.83 (m, 2H, SO2NH2) 7.45 (m, 2H, 2' & 6' ArH) 7.52 (m, 1H, 2" ArH) 7.63 (m, 1H, 6" ArH) 7.74 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 34.46 (1C, 5C, Thiaz) 56.7 (2C, (OCH3)2) 72.33 (1C, 2C, Thiaz) 112.1 (1C, 3" ArC) 113.2 (1C, 6" ArC) 121.2 (2C, 2' & 6' ArC) 122.4 (1C, 2" ArC) 129.3 (2C, 3' & 5' ArC) 132.6 (1C, 1" ArC) 136.6 (1C, 4' ArC) 144.2 (1C, 1' ArC) 148.4 (1C, 4" ArC) 149.5 (1C, 5" ArC) 171.88 (1C, C = O); Mass (m/z): 395.07 (M++1).
4-(2-(4-chlorophenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4h
Orange powder, Yield: 47.55%; MP: 90 oC; Rf: 0.42; IR (KBr, cm-1) : 3405.55 (NH str), 1630 (C = O str), 1384.76 (SO2 str), 618.03 (C-S str), 843.25 (C-Cl str), 1H NMR (500 MHz, δ, ppm): 3.99 (q, 2H, 5-CH2, Thiaz) 6.2 (s,1H, 2-CH, Thiaz) 6.8 (m, 2H, SO2NH2) 7.34 (m, 2H, 2" & 6" ArH) 7.36 (d, 2H, 3" & 5" ArH) 7.63 (d, 2H, 2' & 6' ArH) 7.85 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 31.33 (1C, 5C, Thiaz) 71.49 (1C, 2C, Thiaz) 121.22 (2C, 2' & 6' ArC) 128.28 (2C, 3" and 5" ArC) 128.43 (2C, 3' & 5' ArC) 130.9 (2C, 2" and 6" ArC) 132.1 (1C, 4" ArC) 136.3 (1C, 4' ArC) 136.2 (1C, 1" ArC) 144.3 (1C, 1' ArC) 168.26 (1C, C = O); Mass (m/z): 368.97 (M++1), 370.97 (M++3).
4-(2-(2,4-dichlorophenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4i
Yellow powder, Yield: 57.07%; MP: 110 oC; Rf: 0.54; IR (KBr, cm-1) : 3360.66 (NH str), 1680.92 (C = O str), 1384.52 (SO2 str), 833.81 (C-Cl str), 618.10 (C-S str), 1H NMR (500 MHz, δ, ppm): 4.0 (q, 2H, 5-CH2, Thiaz) 5.94 (s, 1H, 2-CH, Thiaz) 6.86 (s, 2H, SO2NH2) 7.21 (m, 1H, 6" ArH) 7.27 (m, 1H, 5" ArH) 7.5 (m, 2H, 2' & 6' ArH) 7.74 (m, 1H, 3" ArH) 7.79 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 32.69 (1C, 5C, Thiaz) 67.8 (1C, 2C, Thiaz) 100.4 (1C, 1" ArC) 120.6 (2C, 2' & 6' ArC) 126.4 (1C, 5" ArC) 128.4 (2C, 3' & 5' ArC) 130.2 (1C, 3" ArC) 131.9 (2C, 6" ArC) 134.6 (1C, 4" ArC) 135.9 (1C, 2" ArC) 136.4 (1C, 4' ArC) 143.7 (1C, 1' ArC) 171.38 (1C, C = O); Mass (m/z): 402.93 (M++1), 404.92 (M++3).
4-(2-(2,6-dichlorophenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4j
Pale yellow powder, Yield: 44.66%; MP: 138 oC; Rf: 0.43; IR (KBr, cm-1) : 3314.79 (NH str), 3081.21 (Ar-CH str), 1643.96 (C = O str), 1384.73 (SO2 str), 833.56 (C-Cl str), 618.11 (C-S str), 1H NMR (500 MHz, δ, ppm): 4.1 (q, 2H, 5-CH2, Thiaz) 5.8 (s, 1H, 2-CH, Thiaz) 7.38 (m, 2H, SO2NH2) 7.4 (m, 2H, 2' & 6' ArH) 7.52 (m, 1H, 4" ArH) 7.61 (m, 2H 3" & 5" ArH) 7.9 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 32.55 (1C, 5C, Thiaz) 62.43 (1C, 2C, Thiaz) 121.6 (2C, 2' & 6' ArC) 126.73 (2C, 3" and 5" ArC) 128.4 (1C, 4" ArC) 128.9 (2C, 3' & 5' ArC) 135.4 (2C, 2" and 6" ArC) 136.4 (1C, 4' ArC) 140.3 (1C, 1" ArC) 143.86 (1C, 1' ArC) 170.38 (1C, C = O); Mass (m/z): 402.93 (M++1), 404.92 (M++3).
4- (2-(4-bromophenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4k
Orange powder, Yield: 84.74%; MP: 212-214 oC; Rf: 0.44; IR (KBr, cm-1) : 3424.85 (NH str), 1640 (C = O str), 1384.77 (SO2 str), 752.53 (C-Br str), 618.35 (C-S str), 1H NMR (500 MHz, δ, ppm): 4.02 (q, 2H, 5- CH2,Thiaz) 6.47 (s, 1H, 2-CH, Thiaz) 6.93 (s, 2H, SO2NH2) 7.21 (d, 2H, 2" & 6" ArH) 7.53 (d, 2H, 2' & 6' ArH) 7.79 (d, 2H, 3' & 5' ArH) 7.86 (d, 2H, 3" & 5" ArH); 13C NMR (125 MHz, δ, ppm): 32.41 (1C, 5C, Thiaz) 71.52 (1C, 2C, Thiaz) 121.6 (1C, 4" ArC) 121.4 (2C, 2' & 6' ArC) 129.3 (2C, 3' & 5' ArC) 130.9 (2C, 2" and 6" ArC) 131.8 (2C, 3" and 5" ArC) 136.4 (1C, 4' ArC) 138.3 (1C, 1" ArC) 144.6 (1C, 1' ArC) 171.37 (1C, C = O); Mass (m/z): 412.96 (M++1).
4-(2-(4-nitrophenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4l
Pale brownish powder, Yield: 98.59%; MP: 96 oC; Rf: 0.77; IR (KBr, cm-1) : 3380.52 (NH str), 1650 (C = O str), 1596.22 (NO2 Asy str), 1384.77 (SO2 str), 1346.91 (NO2 sym str), 617.93 (C-S str), 1H NMR (500 MHz, δ, ppm): 3.9 (q, 2H, 5-CH2, Thiaz) 6.81 (s, 1H, 2-CH, Thiaz) 7.32 (s, 2H, SO2NH2) 7.59 (m, 2H, 2' & 6' ArH) 7.72 (m, 2H, 2" & 6" ArH) 7.76 (m, 2H, 3' & 5' ArH) 8.16 (m, 2H, 3" & 5" ArH); 13C NMR (125 MHz, δ, ppm): 32.53 (1C, 5C, Thiaz) 61.64 (1C, 2C, Thiaz) 121.38 (2C, 2' & 6' ArC) 123.96 (2C, 3" and 5" ArC) 130.53 (4C, 3', 5', 2" & 6" ArC) 139.96 (1C, 4' ArC) 141.44 (1C, 1', ArC) 147.18 (1C, 1", ArC) 147.29 (1C, 4", ArC) 170.65 (1C, C = O); Mass (m/z): 380 (M++1).
4-(2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)benzenesulfonamide 4m
Pale brownish powder, Yield: 32.65%; MP: 128 oC; Rf: 0.81; IR (KBr, cm-1) : 3385.74 (NH str), 1680 (C = O str), 1385 (SO2 str), 1110.32 (C-F str), 618.28 (C-S str), 1H NMR (500 MHz, δ, ppm): 3.97 (q, 2H, 5-CH2,Thiaz) 6.41 (s, 1H, 2-CH, Thiaz) 6.94 (s, 2H, SO2NH2) 7.14 (m, 2H, 3" & 5" ArH) 7.22 (m, 2H, 2" & 6" ArH) 7.49 (d, 2H, 2' & 6' ArH) 7.8 (d, 2H, 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 33.48 (1C, 5C, Thiaz) 72.9 (1C, 2C, Thiaz) 115.5 (2C, 3" and 5" ArC) 121.4 (2C, 2' & 6' ArC) 129.3 (2C, 3' & 5' ArC) 130.5 (2C, 2" and 6" ArC) 134.6 (1C, 1" ArC) 136.4 (1C, 4' ArC) 143.8 (1C, 1' ArC) 160.7 (1C, 4" ArC) 172.83 (1C, C = O); Mass (m/z): 353.01 (M++1), 354.01 (M++2).
4-(4-oxo-2-styrylthiazolidin-3-yl)benzenesulfonamide 4n
Pale brownish powder, Yield: 24.44%; MP: 202 oC; Rf: 0.70; IR (KBr, cm-1) : 3384.84 (NH str), 1670 (C = O str), 1382 (SO2 str), 618.27 (C-S str), 1H NMR (500 MHz, δ, ppm): 4.4 (q, 2H 5-CH2 Thiaz) 5.7 (d, 1H 2-CH Thiaz) 6.53 (m, 1H CH) 6.88 (d, 1H CH) 6.89 (m, 2H SO2NH2) 7.29 (m, 2H 3" & 5" ArH) 7.30 (m, 1H 4" ArH) 7.44 (m, 2H 2' & 6' ArH) 7.72 (m, 2H 2" & 6" ArH) 7.8 (m, 2H 3' & 5' ArH); 13C NMR (125 MHz, δ, ppm): 34.1 (1C, 5C, Thiaz) 58.5 (1C, 2C, Thiaz) 121.3 (2C, 2' & 6' ArC) 123.2 (1C. CH) 127.8 (1C, 4" ArC) 128.6 (2C, 2" and 6" ArC) 128.8 (2C, 3" and 5" ArC) 129.6 (2C, 3' & 5' ArC) 129.8 (1C. CH) 136.5 (1C, 1" ArC) 136.6 (1C, 4' ArC) 144.7 (1C, 1' ArC) 171.63 (1C, C = O); Mass (m/z): 361.06 (M++1).
Biological activity
DPPH scavenging assay
A stock solution of DPPH (0.1 mM) in ethanol (95%) was prepared. The concentrations of40, 80, 160, 320, and 640 µg of2,3-disubstituted thiazolidin-4-ones 4a-n were prepared in ethanol. For the different concentration sample solutions, 2 mL DPPH stock solution was added and the volume was completed to 4 mL with ethanol. The reaction mixtures were allowed to stand for 30 min in the dark and absorbance was recorded at 517 nm against blank. Ascorbic acid was used as reference. The experiment was carried out in triplicate. The free radical scavenging activity was calculated by
The effective concentration of the sample required to scavenge the DPPH radical by 50% (IC50) was obtained by linear regression analysis between concentration and absorbance [15].
Acute oral toxicity
Acute oral toxicity of 2,3-disubstituted thiazolidin-4-ones (4a-n) was performed as per OECD guidelines 425 [16]. The Swiss albino female mice were fasted before dosing for 18 h. The animals were administered experimental compounds at a dose of 2000 mg/kg b.w. as aqueous suspension of 0.5% Tween 80 orally and observed for 4 h after dosing and for 14 days until death.
Antidiabetic activity
The adult healthy Sprague Dawley rats of either sex was fed 30% D-fructose in water for 30 days and their serum glucose level was estimated using a glucose kit at 505 nm on the 0th, 15th and 30th days. The animals which exhibited hyperglycemia and hyperlipidemia were selected for the study. The rats were allowed to continue to receive fructose until the end of the study. The animals were divided into six groups, each consisting of six as follows: Group 1 (normal): received the water orally, Group 2 (control): received 30% fructose solution and 0.5% aqueous Tween 80 p.o., Group 3 (standard): received 30% fructose solution and pioglitazone (15 mg/kg b.w.) aqueous suspension with 0.5% Tween 80 p.o, and Groups 4, 5, 6, and 7 (Diabetic): received 30% fructose solution, compound 4e (50 mg/kg b.w.) in 0.5% Tween 80 aqueous suspension p.o., compound 4h, 4i, and 4n (200 mg/kg b.w.) in 0.5% Tween 80 aqueous suspension p.o., respectively [17]. Approximately 2 mL of blood was collected and centrifuged at 3500 RPM for 15 min to obtain the serum. The serum glucose was estimated on the 0th, 3rd, 7th, 14th and 21st days. The serum triglyceride and total cholesterol was estimated before and after treatment.
Estimation of serum glucose
The serum glucose was estimated by means of Trinder's method [18]. Glucose reagent 1 mL was added into blank, standard and test. 10 µL of distilled water was appended to blank, standard with 10 µL to standard solution and 10 µL sample serum was added to test. The mixture was mixed well and incubated for 10 min at 37 °C. The absorbance was read against blank at 505 nm. The glucose level was expressed as:
Estimation of total serum cholesterol
The total serum cholesterol was estimated by the CHOD-PAP method [19]. Into each blank, standard and test 1000 µL of working reagent was added. Blank was supplemented with 10 µL distilled water, standard with 10 µL standard solution and test was appended 10 µL sample serum, mixed well and incubated for 10 min at 37 °C. The absorbance was read against blank at 505 nm. The cholesterol level was calculated by:
Estimation of serum triglycerides
The serum triglycerides level was estimated by means of GPO-Trinder's method [20]. Working reagent 1000 µL was added into each blank, standard, and test. Blank was supplemented with 10 µL distilled water, standard with 10 µL standard solution, and test was supplemented with 10 µL sample serum, mixed well and incubated for 10 min at 37 °C. The absorbance was read at 546 nm against blank. Triglycerides level was calculated by:
Histopathology
The rats were anaesthetised and exsanguinated. The pancreas from control, standard, and test groups were fixed with 10% formalin and embedded in paraffin wax and cut into longitudinal sections of 4 mm thickness. The sections were stained with haemotoxylin and eosin dye for histopathological observation.
Molecular docking
The three-dimensional crystal structure of PPAR-γ with PDB ID: 4PRG [22] was downloaded from the RCSB protein data bank. Water molecules were removed followed by the addition of Kollaman’s charges and polar hydrogens using AutoDock 1.5.6 tools [23]. The structures of ligands 4a-n were drawn by ChemDraw 16.0 and copied as SMILES. The Avogadro tool [24] was used to generate the .pdb format of ligand with energy minimisation. The .pdb file was converted into .pdbqt format for input into AutoDock 1.5.6 tools. The grid file was generated and saved as .gpf. The Lamarckian Genetic Algorithm was used to find the conformers with the lowest binding energies and the .dpf file was generated to dock. The final docking task was performed by Autodock vina [25]. The docked file was saved as .dlg format and analysed for the binding interaction of ligand with target in different conformations. The highest binding energy conformation of ligand was selected and its interactions were visualised in Biovia discovery studio 2021 [26].
Physicochemical, drug likeness and ADME properties
The ADME properties and drug likeness of 2,3-disubstituted thiazolidin-4-ones (4a-n) was predicted by Swiss Prediction web tool [27]. The structures of compounds 4a-n and pioglitazone were drawn in ChemDraw 16.0 and copied as SMILES and then submitted one by one to web tool to predict the physicochemical, ADME, and drug likeness properties.
Statistics
The data of animal experiments were expressed as mean ± standard error of mean (SEM) and analysed by means of Graphpad prism version 8.0.0 (Graphpad Software, San Diego, USA) [21]. The statistical difference between the treatments and various groups were tested by analysis of variance (ANOVA), followed by Dunnett's multiples comparison test. Data with p-valué < 0.01 was considered significant
Results and discussion
The hypoglycemic side effect of antimicrobial sulfonamides led to the development of the sulfonyl urea class of hypoglycemic agents such as tolbutamide and chlorpropamide. Glitazone antidiabetics such as rosiglitazone and pioglitazone show their activity due to the presence of acidic thiazolidinedione head. Enthused by this fact, the title compounds which have both the features of sulfonamide and thiazolidin-4-one (that closely resembles the thiazolidinedione present in glitazone antidiabetics) in the same molecule were designed by way of the molecular hybridisation strategy [28] with an anticipation of better hypoglycemic activity [29].
Chemistry
The design and synthesis of the title compounds 2,3-disubstitued thiazolidin-4-ones (4a-n) are illustrated in Figure 1. A series of Schiff bases 3a-n was prepared by condensing substituted aromatic aldehydes with sulphanilamide in absolute alcohol with modérate to excellent yield.
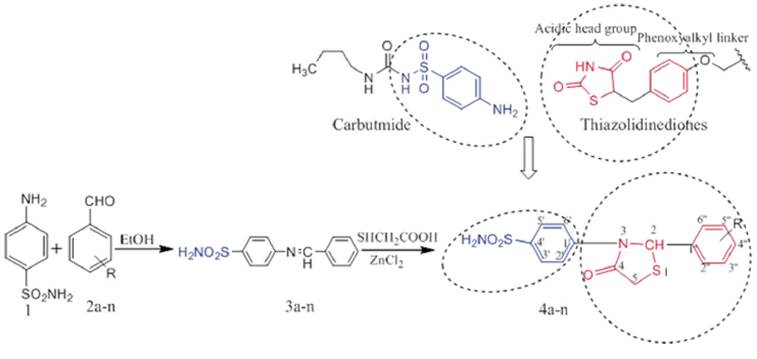
R = a : H; b : 4-CH3; c : 4-OCH3; d : 2-OH; e : 4-OH; f : 4-N(CH3)2; g : 3,4-(OCH3)2; h : 4-Cl; i : 2,4-Cl2; j : 2,6-Cl2 k : 4-Br; l : 4-NO2; m : 4-F.
Figure 1 Design and synthesis of 2,3-disubstituted thiazolidin-4-ones.
The IR spectra of compounds 3a-n showed the characteristic C = N stretching frequency between 1604.77 and 1627 cm-1 among others. Further, compounds 3a-n were reacted with thioglycolic acid in presence of anhydrous zinc chloride to yield compounds 4a-n in modérate to excellent yield. The 2,3-disubstituted thiazolidinones 4a-n displayed the methylene protons of the C-5 thiazolidinone ring at δ 3.9-4.4 ppm as quartet in 1H NMR. The protón of thiazolidinone C-2 resonated at δ 5.7-6.81 ppm among other aromatic protons. The thiazolidinone C-2 appeared at δ ~ 62 ppm while C-5 resonated at δ ~ 33 ppm and C-4 showed signal at δ ~171 ppm amidst other carbons in 13C NMR. Further, the presence of C-S stretching at 617.93-618.80 cm1, S02 stretching at 1382-1385 cm-1 and C = O stretching at 1606-1680.92 cm2 in IR spectrum confirmed their structure. Compounds 4a-n displayed M+ and M++1 ion peaks in their required value in mass spectrum suggesting their formation.
DPPH scavenging assay
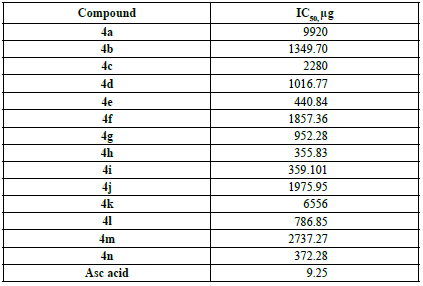
The DPPH radical scavenging activity of 2,3-disubstituted thiazolidin-4-ones 4a-n is presented on Table 1. The DPPH radical scavenging % was calculated as per equation 1. The p-chloro substituted compound 4h exhibited weak DPPH scavenging activity (IC = 355.83 µg) followed by 2,4-dichloro substituted 4i (IC = 359.101 µg), 4n with vinyl group (IC50 = 372.28 µg), and p-hydroxy substituted 4e (IC50 = 440.84 µg) when compared with standard, ascorbic acid (IC50 = 9.25 µg). The remainder of the compounds did not exhibit good DPPH scavenging activity. Compounds with electron attracting substituents exhibited better DPPH scavenging than those with electron releasing substituents.
Acute oral toxicity
Compounds 4e, 4h, 4i, and 4n were found to be safe at the dose of 2000 mg/ kg b.w. Thus, they were tested at 200 mg/kg b.w. and compound 4e at 50 mg/kg b.w. orally for in vivo antidiabetic activity.
Antidiabetic activity
It is a well-known fact that, diabetes mellitus is associated with oxidative stress [30] and compounds having the antioxidant property will be of great value in the treatment of diabetes. Therefore, the thiazolidin-4-ones that have exhibited good antioxidant activity in the DPPH assay namely, 4e, 4h, 4i and 4n were selected for antidiabetic activity. The antidiabetic activity was carried out by estimating the serum glucose level as per equation 2 in rats with fructose induced diabetes. The estimation of the serum glucose level was carried out by Trinder's method in which, glucose oxidase reacts with glucose, water, and oxygen to form gluconic acid and hydrogen peroxide. The enzyme peroxidase catalyses the oxidative coupling of 4-aminoantipyrine with phenol in the presence of hydrogen peroxide to yield a purple coloured quinoneimine complex with absorbance proportional to the concentration of glucose[31].
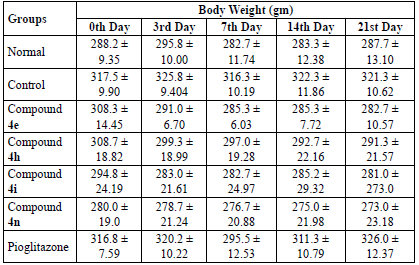
The administration of fructose resulted in the induction of diabetes in rats when compared to the normal group. The tested compounds exhibited highly significant antidiabetic activity when compared with the control group. Compound 4e with p-hydroxy substitution reduced serum glucose by 67.93% followed by compound 4h (p-Cl) by 66.03%, compound 4i (2,4-Cl2) by 65.29%, and compound 4n by 62.68% on the 21st day of the treatment. All the compounds exhibited higher glucose lowering activity than the standard drug pioglitazone. Contrary to DPPH scavenging activity, p-OH substitution seems to favour the activity more than p-Cl and 2,4-Cl2. The reduction in the serum glucose level by way of compounds was found to be significant when compared with pioglitazone on the 3rd day, compound 4e exhibited a significant reduction in the serum glucose level on the 14th day. The results are tabulated on Table 2.
Table 2 Antidiabetic Activity of Compounds 4e, 4h, 4i and 4n.
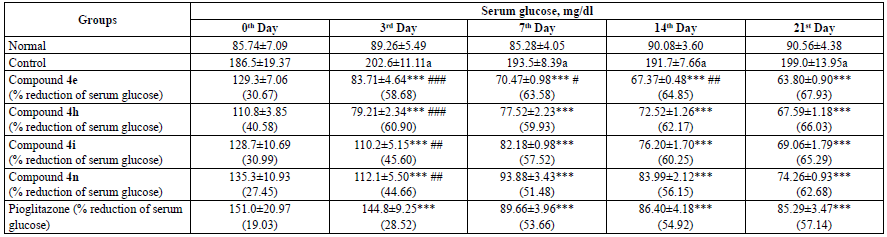
All the values are expressed as mean ± SEM, n = 6, a p < 0.001 as compared to the normal group, ***p < 0.001 as compared to the control group and ### p < 0.001, ## p < 0.01 and # p < 0.05 as compared to the standard group [ANOVA followed by multiple comparison Dunnett's test].
Total serum cholesterol
Cholesterol esterase catalyses the conversion of cholesterol ester to cholesterol in the presence of water. The released cholesterol is then converted into cholest-4-en-3-one and hydrogen peroxide in the presence of oxygen by cholesterol oxidase. The released hydrogen peroxide reacts with 4-aminoantipyrine and phenol to yield a purple complex of quinoneimine that was measured spectrophotometrically [19]. The serum cholesterol was calculated as per equation 3. The tested compounds exhibited better and significant serum cholesterol lowering activity than pioglitazone, when compared with the control group. Compound 4e possessed the highest activity followed by 4h, 4i, and 4n. The activity pattern displayed by the compounds is the same as that of antidiabetic activity. The reduction in total cholesterol by tested compounds was found to be insignificant when compared with pioglitazone; only compound 4e has shown significant activity on the 21st day. The results are tabulated on Table 3.
Table 3 Effect of compounds 4e, 4h, 4i and 4n on serum total cholesterol and triglycerides.
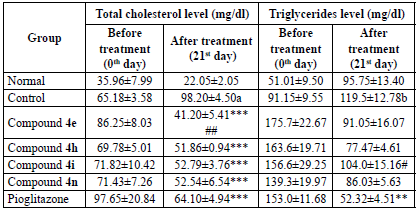
All the values are expressed as mean ± SEM, n = 6, a p < 0.001 as compared to the normal group, ***p < 0.001 as compared to the control group and ##p < 0.01 as compared to the standard group. [One way ANOVA followed by multiple comparison Dunnett's test].
Serum triglycerides
The determination of serum triglycerides was done by means of the GPO-Trinder method. Lipase hydrolyses serum triglycerides to glycerol and free fatty acids. In the presence of ATP and glycerol kinase, the glycerol is converted to glycerol-3-phosphate. The glycerol-3-phosphate is then oxidised by glycerol phosphate oxidase to dihydroxy acetone phosphate and hydrogen peroxide. The condensation of hydrogen peroxide with TOOS reagent (sodium 3-(ethyl(m-tolyl)amino)-2-hydroxypropane-1-sulfonate) and 4-aminoantipyrine yielded a coloured complex of quinoneimine which absorbs at 546 nm [ 20]. The serum triglycerides level was calculated as per equation 4. The tested compounds reduced the triglyceride level after the 21st day. However, it was found to be insignificant, whereas pioglitazone significantly reduced triglycerides when compared to the control group. Compound 4h exhibited the highest serum triglyceride reduction followed by 4n. Compound 4e displayed moderate activity. Compound 4i with 2,4-Cl2 substitution has significantly reduced serum triglycerides after the 21st day when compared with standard pioglitazone. The results are tabulated on Table 3.
Body weight
The tested compounds were found to decrease the elevated body weight when tested at different time intervals. However, it was insignificant when compared with the control and standard groups. The results are tabulated on Table 4.
Histopathology
The histopathological investigation of the pancreas of rats belonging to the normal group showed a normal architecture of islets of Langerhans with pale, rounded and ovoid p cells in the centre embedded in the exocrine portion of the pancreas. Whereas, shrinkage of islets of Langerhans with degeneration and necrosis of cells with their nucleus appearing densely basophilic with predominant karyolysis was observed in rats of the control group. The pancreas of diabetic rats treated with pioglitazone showed a normal architecture of islets of Langerhans with pale, large, round to ovoid shape containing cells embedded in the exocrine portion of the pancreas. In the case of a pancreas treated with compounds 4h and 4i normal sized islets of Langerhans but some degeneration of p cells in the centre were noticed, suggesting a protective role of compounds 4h and 4i on the islets of Langerhans. The histopathological changes are presented in Figure 2.
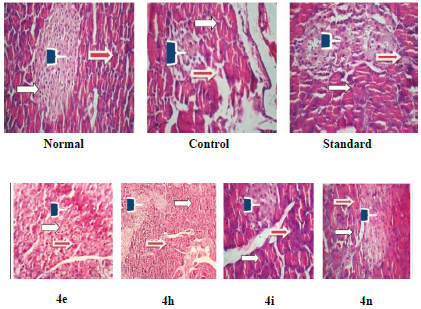
Normal: Pancreatic islet of rat showed intact cluster of β-cells and architecture of islets of Langerhans. Control: Showed destruction of pancreatic islets of β-cells, islet shape and number of islets are reduced. Standard: Showed apparently reduced degranulation architecture, moderately restored pancreatic cells, islet shape and increased number of islets. Treatment: 4e, 4h, 4i and 4n treated showing regeneration of β-cells, restoration of architecture, shape and increase in the number of islets.
Figure 2 Effect of 2,3-disubstitued thiazolidin-4-ones on pancreatic cell.
Molecular docking
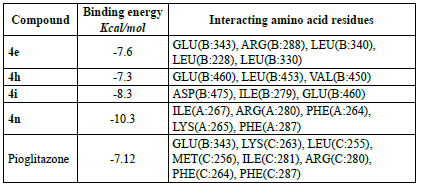
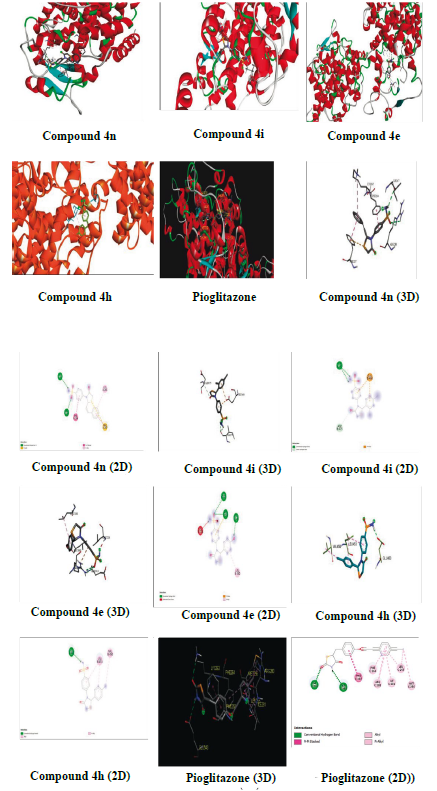
The compounds that were tested for antidiabetic activity were docked with PPAR-y (PDB ID: 4PRG) using autodock tools 1.5.6 to explore their interaction. The PPAR-y plays an important role in the regulation of glucose and homeostasis and was used as a molecular target for the 2,4-thiazolidinedione class (glitazones) of antidiabetic agents [32]. The binding energies and amino acid residues with which compounds 4e, 4h, 4i, 4n and pioglitazone interacted are presented on Table 5 and in Figure 3. The compounds exhibited more binding energy with PPAR-y than with pioglitazone.
It is interesting that, the tested compounds exhibited higher binding energy with PPAR-y. The compound 4n demonstrated the highest binding energy with -10.3 kcal/mol among the tested compounds and formed two hydrogen bonds with amino acid residues ILE (A:267) and ARG (A:280), and also formed other bonds by interacting with other amino acid residues PHE (A:264), LYS (A:265), and PHE (A:287). Likewise, compound 4i showed good binding energy of -8.3 kcal/mol and formed two hydrogen bonds with ASP (B:475) and ILE (B:279), and also formed another bond with GLU (B:460). Compound 4e displayed good binding energy of -7.6 kcal/mol and formed three hydrogen bonds with GLU (B:343), ARG (B:288), and LEU (B:340), and formed other bonds with LEU (B:228) and LEU (B:330). Compound 4h also showed binding energy of -7.3 kcal/mol and formed a hydrogen bond with GLU (B:460), and other bonds with LEU (B:453) and VAL (B:450). Compound 4n interacted with the A chain while, others with the B chain of PPAR-y. All the results are compared with pioglitazone which had binding energy of -7.12 kcal/mol and formed two hydrogen bonds with GLU (B:343) and LYS (C:263), and other bonds with PHE (C:287), LEU (C:255), MET (C:256), ILE (C:281), PHE (C:264), and ARG (C:280) with the B and C chains. Thus, compound 4n exhibited excellent binding energy and interaction with PPAR-y followed by 4i, 4e and 4h when compared with pioglitazone. It is interesting to note that, the compounds which showed good antidiabetic activity interacted with a lower binding energy with PPAR-y.
Physicochemical, ADME, and drug likeness properties
For a molecule to be effective as a potent drug, it must reach its target in the body in sufficient concentration and stay there in a bioactive form long enough for the expected biologic events to occur. Drug development involves assessments of pharmacokinetic properties such as absorption, distribution, metabolism, and excretion (ADME); in this context computer models constitute valid alternatives to experiments. The Swiss ADME web tool provides easy efficient inputs and free access to a pool of past yet robust predictive models for the physicochemical properties, pharmacokinetics, and drug likeness of compounds. The ADME and drug likeness of 2,3-disubstitued thiazolidin-4-ones (4a-n) were determined by the Swiss ADME web tool and the results are tabulated on Table 6.
Table 6 In silico parameters of 2,3-disubstituted thiazolidin-4-ones (4a-n).
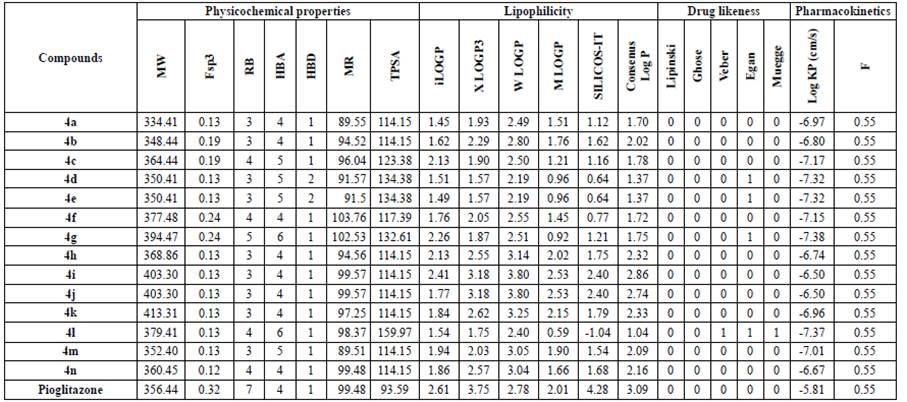
MW: Molecular Weight, Fsp3: Fraction of sp3 Carbon atoms, RB: Rotatable Bonds, HBA: Hydrogen Bond Acceptor, HBD: Hydrogen Bond Donor, MR: Molar Refractivity, TPSA: Topological Polar Surface Area, LogP: Indicator of Lipophilicity, Log KP: Skin Permeation, F: Bioavailability Score
The filters used to evaluate the drug likeness of the title compounds were i) Lipinski filter: MW≤500, MLogP≤ 4.15, HBA≤ 10, HBD≤ 5 [33] ii) Ghose filter: 160≤ MW≤ 480, -0.4≤ WlogP≤ 5.6, 40≤ MR≤ 130, 20≤ atoms≤ 70 [34] iii) Veber (GSK) filter: RB≤ 10, TPSA≤ 140 [35] iv) Egan (Pharmacia) filters: WLogP≤ 5.88, TPSA≤ 131.6 [36] v) Muegge (Bayer) filter : 200≤ MW≤ 600, -2≤ XLogP≤ 5, TPSA≤ 157; HBD≤ 5, RB≤ 15, number of rings ≤ 7; number of carbons >4, number of heteroatoms ≤ 1 [37]. The compounds did not violate the Lipinski and Ghose rule but, some violated the Veber, Egan, and Muegge rules. To summarise, 2,3-disubstituted thiazolidin-4-ones met the criteria of drug likeness and exhibited satisfactory ADME parameters.
Conclusions
A series of 2,3-disubstituted thiazolidinones 4a-n were synthesised from Schiff base intermediates 3a-n that have been derived from sulfanilamide. The final products were characterised by physicochemical parameters and spectral data.
Compounds 4e, 4h, 4i, and 4n exhibited weak DPPH radical scavenging activity. The acute toxicity study revealed the safety of compounds 4e, 4h, 4i and 4n at the dose of 2000 mg/kg b.w. p.o. Compounds 4h, 4i and 4n were tested at 200 mg/kg b.w. and compound 4e at 50 mg/kg b.w. orally for in vivo antidiabetic activity. Compounds 4e with p-hydroxy substitution, 4h with p-chloro substitution, 4i with 2,4-dichloro substitution, and 4n with vinyl substitution exhibited highly significant antidiabetic activity when compared with the control group. The tested compounds exhibited better and significant serum cholesterol lowering activity with reference to the control group. Compound 4e possessed the highest cholesterol lowering activity followed by 4h, 4i, and 4n. The tested compounds also reduced the serum triglyceride level after the 21st day, however this was found to be insignificant in comparison to the control group. Compound 4h exhibited the highest serum triglyceride reducing activity followed by 4n and 4e. The tested compounds reduced elevated body weight but it was found to be insignificant when compared with the control group. The histopathology demonstrated the protective role of compounds 4h and 4i on the p cells of islets of Langerhans. The molecular docking showed compound 4n binds with high energy more than pioglitazone with PPAR-y. The physicochemical, ADME, and drug likeness properties of most of the synthesised compounds were found to be within permitted limits. Thus, the molecular architecture developed and synthesised in this study would be of great interest with good antidiabetic potential warranting further work.