Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Agronómica
Print version ISSN 0120-2812
Acta Agron. vol.65 no.3 Palmira July/Sep. 2016
https://doi.org/10.15446/acag.v65n3.51106
http://dx.doi.org/10.15446/acag.v65n3.51106
Tree seedling growth promotion by dual inoculation with Rhizoglomus fasciculatum (Thaxt.) Sieverding, Silva & Oehl and Mortierella sp., rhizosphere fungi for reforestation purposes, to promote plant P uptake and growth at the nursery state
Promoción de crecimiento de plántulas forestales por doble inoculación con los hongos rizosféricos Rhizoglomus fasciculatum (Thaxt.) Sieverding, Silva & Oehl y Mortierella sp., para propósitos de reforestación, promoción de crecimiento y asimilación de fósforo en etapa de vivero
Josaly Moreno, Juan Diego León and Nelson Walter Osorio*
Universidad Nacional de Colombia sede Medellín, Antioquia, Colombia. *Author for correspondence: nwosorio@gmail.com
Rec.:08.06.2015 Acep.: 02.09.2015
Abstract
One of the most promising techniques to promote seedling growth of tree species at nursery is the use of rhizosphere microorganisms. This is particularly relevant in enhancing plant performance in reforestation of degraded lands. A series of experiments were conducted to evaluate the effectiveness of either individual inoculation with the arbuscular mycorrhizal fungus Rhizoglomus fasciculatum (Thaxt.) Sieverding, Silva & Oehl or dual inoculation with R. fasciculatum and the phosphate solubilizing fungus Mortierella sp., at two doses (50 and 100 kg of inoculum per m3 of substrate), on plant growth and phosphorus (P) uptake of Tecoma stans, Dodonaea viscosa, Fraxinus chinensis, and Lafoensia speciosa. These plant species are commonly used in Colombia in reforestation programs. The results indicated that treatments were effective to increase plant performance of the four plant species; however, the effect was significantly higher when both fungi R. fasciculatum and Mortierella sp. were concomitantly inoculated than when only the mycorrhizal fungus was applied. Overall, the dual inoculation at the dose of 50 kg m-3 had higher or similar effects than at 100 kg m-3. The results suggest that the dual inoculation was more effective in order to prevent plant P deficiency and stimulate plant growth.
Keywords: Andisol, forestry nursery, Mortierella, phosphorus, Rhizoglomus fasciculatum.
Resumen
Una de las técnicas más importantes para promover el crecimiento vegetal en el invernadero de especies forestales es el uso de microorganismos rizosféricos. Esto es particularmente relevante para mejorar el desarrollo vegetal en programas de reforestación de tierras degradadas. Una serie de experimentos se realizaron para evaluar la efectividad de inoculaciones individuales y combinadas con el hongo micorrícico arbuscular Rhizoglomus fasciculatum (Thaxt.) Sieverding, G.A. Silva & Oehl y el hongo solubilizador de fosforo (P) Mortierella sp., a dos dosis (50 y 100 kg de inóculo por m3 de sustrato) sobre el crecimiento y absorción de P de Tecoma stans, Dodonaea viscosa, Fraxinus chinensis y Lafoensia speciosa. Estas especies vegetales son comúnmente usadas en Colombia en programas de reforestación. Los resultados indican que los tratamientos fueron efectivos para incrementar el crecimiento y la absorción de P de las cuatro especies; sin embargo, el efecto fue significativamente mayor cuando ambos hongos R. fasciculatum y Mortierella sp., fueron conjuntamente inoculados que cuando se usó sólo R. fasciculatum. En general, la inoculación dual en una dosis de 50 kg m-3 tuvo efectos significativamente mayores o similares que la dosis de 100 kg m-3. Estos resultados sugieren que la doble inoculación fue más efectiva para prevenir la deficiencia de P y estimular el crecimiento vegetal.
Palabras clave: Andisol, fósforo, Mortierella, Rhizoglomus fasciculatum, vivero forestal
Introduction
Soil degradation is the result of soil mismanagement that reduces soil productivity and environmental services (Herrero et al., 2010). The most common factors causing land degradation and thus threatening ecosystem services in developing countries are soil erosion, deforestation, overgrazing, overtillage (Lal, 2009).
An alternative to improve soil quality of these degraded lands is the establishment of new forest plantations, agroforestry, and silvopastoral systems. This active restoration model accelerates the restablishment of ecosystem functioning through the activation of soil biogeochemical cycling of nutrients and carbon sequestration that improves soil quality (Restrepo, Flórez, León & Osorio, 2013).
However, a factor restricting the successful establishment of new plantations in degraded land is the lack of enough soil available phosphate (P). Plant species included in the active restoration model must overcome this P deficiency in order to achieve a satisfactory growth and development (Sousa Franco, Oliveira, & Castro, 2009).
An alternative consists of using arbuscular–mycorrhizal fungi and phosphate solubilizing microorganisms (Zangaro, Nisizaki, Domingos, & Nakano, 2003), as early as the nursery stage. This not only may favor the production of vigorous plants, but also provide root symbionts that represent a competitive advantage to plants to improve their establishment and survival in the degraded field (Salifu & Timmer, 2001).
Although there is a great potential for the effective use of these microorganisms, more studies are required to know the benefits of dual inoculations and different dose of inocula on plant performance at nursery, particularly in those plant species with high potential to be used in restoration of degraded land commonly with low soil fertility. Based on that, the current study was conducted to evaluate the P uptake and growth responses at nursery of four tree species commonly used in tropical highlands for reforestation of watersheds and restoration of degraded lands, to inoculation with fungi: i. individual inoculation with an arbuscular–mycorrhizal fungus (R. fasciculatum); and ii. Dual inoculation with this fungus and a phosphate solubilizing fungus Mortierella sp.
Material and methods
Study area
The study was conducted in a forest nursery (6°15'27''N, 75°30'07''W) located in Santa Elena (Medellin–Antioquia, Colombia) (altitude: 2468 m.a.s.l, mean temperature: 15°C and rainfall regime 1948 mm/year).
Growth substrate
The substrate consisted of a 4:1 volumetric mixture with a soil sample (A horizon, 0–25 cm) and sand. The soil sample was collected from an Andisol (Alic Melanudand) and then passed through a sieve (3 mm aperture size). Soil samples were analyzed in the Laboratory of Biogeochemistry of the Universidad Nacional de Colombia at Medellín – Antioquia, Colombia, with the following results: pH 5.3 (1:2 volumetric ratio), organic matter content 110 g kg-1 (Walkley–Black), P 2 mg kg-1 (Bray No.2); exchangeable Al, Ca, Mg, and K 0.3, 2.9, 0.9 and 0.2 cmolc kg-1 (ammonium acetate), respectively; total N 6 g kg-1 (Kjeldahl); Fe, Mn, Cu and Zn 160, 3, 1, and 6 mg kg-1 (Olsen–EDTA), respectively; B 0.5 mg kg-1 (hot water). The soil was disinfected with Basamid® [active ingredient: dazomet (tetrahydro–3,5–dimethyl–2H–1,3,5–thiadiazine–2–thione)] in order to eliminate plant pathogens at a rate of 200 g m-3. The substrate was covered with a plastic sheet for 10 days and then aerated for 5 days to eliminate any residue. After that, the substrate was amended with the fertilizer grade 10–30–10 at a rate of 3 kg m-3.
Treatments
The substrate received either: (i) arbuscular–mycorrhizal (M) inoculum alone that contained infective propagules of the fungus R. fasciculatum (300 spores per g, mycelia and colonized roots of Brachiaria decumbens grass) suspended in a soil: sand matrix (3:1), or (ii) dual inoculum (MF) composed by the same mycorrhizal inoculum and the P–solubilizing fungus Mortierella sp. (105 colony forming units per g of inoculum). Mortierella sp. was previously cultivated in Potato–Dextrose–Agar medium for 7 days at 28°C, and then suspended in distillated and sterile water. Both fungi were obtained from the collection of the Laboratory of Biogeochemistry at the Universidad Nacional de Colombia at Medellin. Both inocula (M and MF) were applied at two doses (50 or 100 kg m-3). An uninoculated treatment was included as control.
Plant material
Seeds of the plant species Tecoma stans (L.) Juss. Ex. Kunth, Dodonaea viscosa L. Jacq., Fraxinus chinensis Roxb, and Lafoensia speciosa (Kunth) D.C. were allowed to germinate in sterile sand for 7 days, and then two germinated seeds were separately transplanted in each plastic bag. Thirty days later one seedling was removed. Seedlings were grown at the nursery for 150 days, during this time the substrate was watered to maintain it at 50–60% of the maximum water holding capacity. Every 30 days plants were sprayed with the fertilizer Wuxal® (20–0–15) at a concentration of 1 g L-1.
Variables
At the end of the growth period the plant height and stem collar diameter were measured. Also, the shoot dry weight was measured after oven–drying the plant material at 60°C per 72 h. The shoot P content was measured using subsamples of plants, which were ashed in a muffle furnace at 500°C for 3 h and then dissolved first with 1 mL of 1 M HCl and 9 mL of distillated water. In this solution, the P concentration was measured by the molybdate–blue method. Mycorrhizal colonization was determined in fine–roots fragments after clearing with 10% KOH and staining with 0.015% fuchsine acid then, the extent of the mycorrhizal colonization was measured with the gridline intercept method. Also, the presence of the fungus Mortierella sp. in the fine roots was measured with the method developed by Osorio & Habte (2013).
Experimental design and data analysis
Each plant species was considered in an independent experiment with a completely randomized design. Treatments consisted of either individual inoculation with R. fasciculatum (M) or dual inoculation with R. fasciculatum and Mortierella sp. (MF), both inocula in two doses (50 and 100 kg m-3). An uninoculated treatment was included as control. Each treatment had 20 replicates. Data were subjected to analysis of variance and the multiple range test of Duncan for mean separation. Both tests were conducted with a significance level (P) < 0.05 with the software Statgraphics Centurion version XV.
Results and Discussion
As it has been stated (Schroth, & Krauss, 2006), the low soil P availability seems to be one of the most critical issues for land reclamation in the tropics; in order to manage this problem the coinoculation with mycorrhizal fungi and phosphate solubilizing microorganisms may help to reduce this limitation. In fact, our results showed clearly that there was a positive response in plant P uptake and growth of all four plant species to dual inoculation with the arbuscular mycorrhizal fungus R. fasciculatum and the phosphate solubilizing fungus Mortierella sp. The fungus Mortierella sp. can dissolve native or applied insoluble P compounds (e.g., rock phosphate), while the mycorrhizal fungus absorbs P more efficiently than the root does. This produces an additive effect of both microorganisms on plant performance (Osorio & Habte, 2015; Osorio, Habte, & León, 2015). In general, for most of the variables with the dual inoculation the lower dose (50 kg m-3) was the best treatment or at least similar to the higher dose. The results are presented separately by plant species.
Tecoma stans
With plants of T. stans the dual inoculation (MF) with 50 and 100 kg m-3 increased plant height respect to the uninoculated control (7.98 cm) by 108 and 116%, respectively. The individual inoculation also increased the plant height but in lesser proportion 16 and 37% for 50 and 100 kg m-3 doses, respectively (Figure 1A). The dual inoculation (MF) increased significantly the stem collar diameter by 34 and 40% regarding the control (3.2 mm) with the respective doses (i.e. 50 and 100 kg m-3) but there were not significant differences between these values (Figure 1B). The individual inoculation with the higher dose also increased significantly the stem collar diameter by 20%, but it did not happen with the lower dose. The shoot dry weight (SDW) of uninoculated plants had a mean value of 0.40 g/plant, the individual inoculation at 100 kg m-3 increased it by 119%, but it did not happen with the 50 kg m-3 dose (Figure 1C). On the other hand, the increase was even significantly higher with the dual inoculation, 318 and 333% with 50 and 100 kg m-3, respectively. The amount of shoot P was also increased respect to the control plants (0.83 mg P/plant) only with the dual inoculation (MF), 128% and 425% with 50 and 100 kg m-3, respectively (Figure 1D).
Dodonaea viscosa
The plant height of D. viscosa was increased by 105 and 87% with the dual inoculum (MF) with 50 and 100 kg m-3, respectively (Figure 2A). By contrast, there were not significant effects in plant height with the individual inoculation (Figure 2B). The stem collar diameter was only significantly increased with the dual inoculum with the dose of 50 kg m-3. The shoot dry weight increased over the control plants (0.54 g/plant) by 235 and 102% with the dual inoculum (50 and 100 kg m-3, respectively) (Figure 2C). The shoot P content also increased with the dual inoculation (50 kg MF m-3: 369%; 100 kg MF m-3: 223%) respect to the uninoculated control (0.72 mg P/ plant) (Figure 2D).
Fraxinus chinensis
F. chinensis inoculated plants, were significantly higher than the uninoculated plants (9.7 cm). With the individual inoculum, the mean increase was 17% (regardless the dose) and with the dual inoculation it was 37% (regardless the dose) (Figure 3A). The stem collar diameter was significantly increased with the higher dose of the individual inoculum by 39% and even more with the dual inoculum (65 and 62% for 50 and 100 kg m-3 doses, respectively) (Figure 3B). On the other hand, the shoot dry weight was only increased respective to control plants (0.39 g/plant) with the dual inoculum by 260 and 202% with 50 and 100 kg m-3, respectively (Figure 3C). The increase in shoot P content followed the same response pattern, uninoculated plants had a value of 0.83 mg P/ plant and with the dual inoculation the increases were 276 and 404%, respectively (Figure 3D).
Lafoensia speciosa
Uninoculated plants of L. speciosa exhibited a plant height of 4.4 cm, while the inoculated plants were significantly higher by 75%, regardless inoculum type and dose (Figure 4A). The stem collar diameter of uninoculated plants was 1.0 mm and those inoculated was increased by 94% (again regardless inoculum type and dose) (Figure 4B). On the other hand, the shoot dry weight of uninoculated plants was 0.036 g/plant and that of inoculated ones ranged between 0.103 and 0.308 g/plant without significant differences among them respect to inoculum type and dose (Figure 4C). Regarding the shoot P content, the uninoculated plants had a value of 0.065 mg/plant, with the higher dose of the individual inoculum and with both dose of the dual inoculum it was significantly higher and ranged between 0.58 and 0.84 mg P/plant (Figure 4D).
The study of nutrient requirements in the nursery has had increasing interest, being the use of fertilizers the most traditional approach. In addition, from the integrated plant nutrient management perspective the use of biofertilizers has been receiving increasing attention (Pellegrino & Bedini, 2014; Aguirre–Medina, Culebro–Cifuentes, Cadena–Iñiguez, & Aguirre–Cadena, 2014). This is the result of the acknowledgement of the benefits and advantages of symbiotic association between plants and some soil microorganisms. This allowed their use in nursery and plantation practices, for instance, ectomycorrhizal fungi and conifers such those of genus Pinus (Castrillón, León, Osorio, & Carvajal, 2015) and other tropical plant species such as Quercus humboldtii (Fernández, Marchelli, & Fontenla, 2013).
Some nursery experiments with tropical tree species employed in restoration activities have been carried out evaluating their arbuscular mycorrhizal dependency. Most of their results have showed that when soil soluble P concentration is lower than 0.02 mg L-1, tree species respond significantly to mycorrhizal colonization, consequently, an enhancement on growth of the tree species is obtained. By the opposite, as soil soluble P increases up to 0.1–0.2 mg L-1, the dependency of plants on arbuscular mycorrhizal fungi for P uptake diminishes progressively, and tree growth is constrained. In assessing the biometric response at nursery of tree seedlings to inoculation with Glomus aggregatum, Sierra, Castro, & Osorio (2012), did not find increases in the shoot dry weight of Calophyllum brasiliense (mycorrhizal independent) in the inoculated seedlings. In contrast, Sierra, Castro, & Osorio (2015), significant increases in both leaf P content and shoot dry weight, at a 0.02 mg L-1 level in soil solution P, with the mycorrhizal inoculation of seedlings of Nageia rospigliosii, and Senna pistaciifolia Kunth. (Moderately dependent), respectively.
Other studies at nursery have allowed to determine the arbuscular mycorrhizal dependency category of tree species used in tropical forestry and agroforestry. In this way, several authors have reported a very high mycorrhizal dependency for Luehea grandiflora, Senna spectabilis, Tabebuia serratifolia, Sapindus saponaria (Siqueira & Saggin–Junior, 2001), Leucaena leucocephala (Habte & Manjunath, 1991), and Bidens sandvicens (Gemma, Koske, & Habte, 2002).
It has been demonstrated that plant species commonly used in forestry also responded positively to both ectomycorrhizal and endomycorrhizal fungi. This is the case of plants of the Acacia and Eucalyptus genera (Founoune, Duponnois, Ba, & El–Bouami, 2002; Yuan, Huang, Li, & Christie, 2004).
Among biofertilizers with potential use in forestry nursery and plantation are the mycorrhizal fungi and phosphate solubilizing microorganisms, such as those used in this research. Both, constitute an attractive alternative to promote plant performance not only at the nursery but also at plantations in the field (Zaidi et al., 2009). In fact, in most of the cases the level of soil fertility severely limits plant growth and development, particularly in degraded land areas and in abandoned agricultural fields. Under this stage, the use of mycorrhizal fungi at the nursery will provide competitive advantages for plant survival and performance in the field given the increased ability to uptake more nutrients and water that the non–mycorrhizal plants and to protect against root pathogens (Zangaro, Nisizaki, Domingos, & Nakano, 2003).
It is widely reported that P is one of the most limiting plant nutrients, particularly in highly weathered soils of the tropics and in volcanic ash soils, as that used in this study (Schroth & Krauss, 2006). Sepúlveda, Díez, Moreno, León, & Osorio (2014) found that Quercus humboldtii absorbed more P with mycorrhizal inoculation in a volcanic soil characterized by its high P sorption capacity. Comparable results have been found in similar soils with other tropical forestry plant species (Zangaro, Nisizaki, Domingos, & Nakano, 2003).
It is expected that the individual mycorrhizal inoculation at planting overcome the plant P deficiency. However, in some cases this was not enough because the soil P available level was too low (Habte, 2006). As alternative, the dual inoculation involving a fungus capable of dissolving insoluble P compounds may contribute to produce more soil available P and promote the mycorrhizal effects (Turner, Frossard, & Oberson, 2006). Osorio & Habte (2013) found a positive response of Leucaena leucocephala seedlings in a P–deficient Oxisol when Mortierella sp. and Glomus fistulosum were concomitantly inoculated; in this case, the oxalic acid produced by the former dissolved the rock phosphate applied and thus the mycorrhizal fungus had access to more P. Besides, Mortierella sp. is capable of desorbing fixed P from the surface of minerals (Osorio & Habte, 2014), which may also contribute to increase the benefits of the mycorrhizal association on plant P uptake.
Both tested fungi were established in the roots likely as endophytes; some species of Mortierella have been reported as root endophytes in healthy plant. Although they appeared to interact competitively for root colonization sites, the effect they had on plant P uptake and growth was significantly better if they were present together than if R. fasciculatum was present alone. This may be the result of complementary roles that these fungi have on soil P use (P availability and uptake) that can offset the space competition between both and finally produce benefits on host plants. Thus, Mortierella sp. increases soluble P via rock phosphate dissolution whereas R. fasciculatum absorbs efficiently the P released, which is then transferred to the root cells (Osorio & Habte, 2013). This fungal cooperation allows the host plant to capture more P, the most limiting nutrient in tropical soil systems, and consequently promote plant growth; this in turn will favor the allocation of more carbon for both fungal endophytes in the root system (Fellbaum et al., 2014).
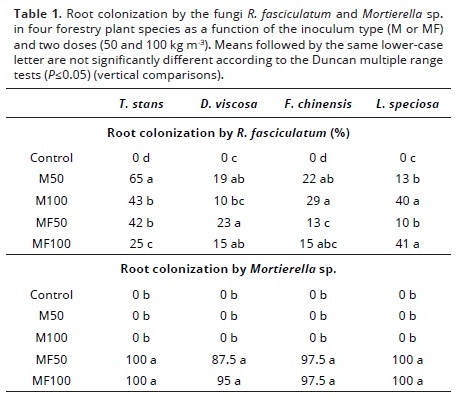
It is worth to mention that uninoculated plants did not exhibit fungal colonization in their roots, while those that received the individual inoculum (M) developed typical mycorrhizal structures (internal mycelia, arbuscules, vesicles); the extent of this mycorrhizal colonization was variable and depended on the amount of inoculum applied and the plant species involved (10–65%) (Table 1). On the other hand, uninoculated plants and inoculated with the individual inoculum (R. fasciculatum) did not show colonization by Mortierella sp. in their roots; those that received dual inoculation had values of root colonization by Mortierella sp. that ranged between 87.5 and 100% (Table 1). Plant species differ in their ability to form the mycorrhizal association and in the extent to which the fungus colonize the root tissues. This is likely associated to the capacity of the root to release flavonoids that act as signals between plants and fungus (Scervino et al., 2005; Seddas et al., 2009). However, it is clear that the value of the mycorrhizal colonization is not an indicator of the plant mycorrhizal dependency and/or the effectiveness of the fungus to promote plant nutrient uptake and growth (Siqueira & Sagging–Junior, 2001). Since both fungi are root endophytes they can compete for root space, but in despite of this competition they can have additive effects when both are concomitantly inoculated (Osorio & Habte, 2013).
Conclusions
- The results showed here for these plant species demonstrate the significance of using dual inoculation as biofertilization to promote plant P uptake and growth at the nursery state and to presumably obtain a better plant development in field conditions. It is clear that the use of arbuscular mycorrhizal fungi can improve plant performance, but such effects can be consistently improved if the P solubilizing fungus can be co–inoculated.
Acknowledgements
We thank Diomer Carvajal and Nicolas Álvarez for supplying plant materials and facilities of the Santa Elena forestry nursery for this research.
References
Aguirre–Medina. J. F. Culebro–Cifuentes. F. Cadena–Iñiguez. J. Aguirre–Cadena. J. F. (2014). Tabebuia donnell–smithii Rose growth inoculated with mycorrhizal fungi and Azospirillum brasilense. Agrociencia, 48(3), 331–345. [ Links ]
Castrillón. M. León. J.D. Osorio. N.W. & Carvajal. D. (2015). Effectiveness of single and combined ectomycorrhizal inocula on three species of pinus at nursery. Commun Soil Sci Plan, 46(2), 169–179. doi: 10.1080/00103624.2014.967856. [ Links ]
Fellbaum. C.R. Mensah. J.A. Cloos. A.J. Strahan. G.E. Pfeffer. P.E. Kiers. E.T. & Bucking. H. (2014). Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol, 203(2), 646–656. doi: 10.1111/nph.12827. [ Links ]
Fernández. N. Marchelli. P. & Fontenla. S. (2013). Ectomycorrhizas naturally established in Nothofagus nervosa seedlings under different cultivation practices in a forest nursery. Microbial Ecol, 66(3), 581–592. doi: 10.1007/s00248–013–0229–9. [ Links ]
Founoune. H. Duponnois. R. Ba. A.M. & El–Bouami. F. (2002). Influence of the dual arbuscular endomycorrhizal / ectomycorrhorizal symbiosis on the growth of Acacia holosericea (A. Cunn. Ex G. Don) in glasshouse conditions. Ann For Sci, 59(1), 93–98. doi: 10.1051/forest: 2001008. [ Links ]
Gemma. J.N. Koske. R.E. & Habte. M. (2002). Mycorrhizal dependency of some endemic and endangered Hawaiian plant species. Am J Bot, 89(2), 337–345. doi: 10.3732/ajb.89.2.337. [ Links ]
Habte. M. (2006). The roles of arbuscular mycorrhizas in plant and soil health. En: N. Uphoff (Ed.), Biological approaches to sustainable soil systems (pp. 129–147). Boca Raton, United States: CRC Press. [ Links ]
Herrero. M. Thornton. P.K. Notenbaert. A.M. Wood. S. Msangi. S. Freeman. H.A. & Rosegrant. M. (2010). Smart investments in sustainable food production: revisiting mixed crop–livestock systems. Science, 327(5967), 822–825. doi: 10.1126/science.1183725. [ Links ]
Lal. R. (2009). Soil degradation as a reason for inadequate human nutrition. Food Sec, 1(1), 45–57. doi: 10.1007/s12571–009–0009–z. [ Links ]
Osorio. N.W. & Habte. M. (2013). Synergistic effect of a phosphate solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in an oxisol fertilized with rock phosphate. Botany, 91(4), 274–281. doi: 10.1139/cjb–2012–0226. [ Links ]
Osorio. N.W. & Habte. M. (2014). Soil phosphate desorption induced by a phosphate solubilizing fungus. Commun Soil Sci Plan, 45(4), 451–460. doi: 10.1080/00103624.2013.870190. [ Links ]
Osorio. N.W. & Habte. M. (2015). Effect of a phosphate–solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in tropical soils with contrasting phosphate sorption capacity. Plant Soil, 389(1–2), 375–385. doi: 10.1007/s11104–014–2357–5. [ Links ]
Osorio. N.W. Habte. M. & León. J.D. (2015). Effectiveness of a rock phosphate solubilizing fungus to increase soil solution phosphate impaired by the soil phosphate sorption capacity. Rev Fac Nac Agron, 68(2), 7627–7636. doi: 10.15446/rfnam.v68n2.50950. [ Links ]
Pellegrino. E. & Bedini. S. (2014). Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol Biochem, 68, 429–439. doi:10.1016/j.soilbio.2013.09.030. [ Links ]
Restrepo. M.F. Flórez. C.P. Osorio. N.W. & León. J.D. (2013). Passive and active restoration strategies to activate soil biogeochemical nutrient cycles in a degraded tropical dry land. Soil Sci, 2013(2013), 1–6. doi: 10.1155/2013/461984. [ Links ]
Rilling. M.C. Wright. S.F. Nichols. K.A. Schmidt. W.F. & Torn. M.S. (2001). Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil, 233(2), 167–177. doi: 10.1023/A: 1010364221169. [ Links ]
Salifu. K.F. & Timmer. V.R. (2001). Nutrient retranslocation response of Picea mariana seedlings to nitrogen supply. Soil Sci Soc Am J, 65(3), 905–913. doi: 10.2136/sssaj2001.653905. [ Links ]
Schroth. G. & Krauss. U. (2006). Biological soil fertility management for tree–crop agroforestry. En N. Uphoff (Ed.), Biological approaches to sustainable soil systems (pp. 291–303). Boca Raton, United States: CRC Press. [ Links ]
Scervino. J.M. Ponce. M.A. Erra–Bassells. R. Vierheilig. H. Ocampo. J.A. Godeas. A. (2005). Flavonoids exclusively present in mycorrhizal roots of white clover exhibit a different effect on arbuscular mycorrhizal fungi than flavonoids exclusively present in non–mycorrhizal roots of white clover. J Plant In, 1(1), 15–12. doi: 10.1080/17429140500192597. [ Links ]
Seddas. P.M. Arias. C.M. Arnould. C. Van Tuinen. D. Godfroy. O. Benhassou. H.A. Gouzy. J. Morandi. D. Dessaint. F. Gianinazzi–Pearson. V. (2009). Symbiosis–related plant genes modulate molecular responses in an arbuscular mycorrhizal fungus during early root interactions. Mol Plant Microbe In, 22(3), 341–351. doi: 10.1094/MPMI–22–3–0341. [ Links ]
Sepúlveda. Y.L. Díez. M.C. Moreno. F.H. León. J.D. & Osorio. N.W. (2014). Efectos de la iluminación relativa y la fertilización sobre el crecimiento de plántulas de roble andino en vivero. Acta Biol Col, 19, 211–220. [ Links ]
Sierra. J.A. Castro. D. & Osorio. W. (2012). Mycorrhizal dependence of barcino (Clusiaceae: Calophyllum brasiliense Cambess). Actualidades Biológicas, 34, 199–206. [ Links ]
Sierra. J.A. Castro. D. & Osorio. W. (2015). Mycorrhizal dependency of Alcaparro (Senna pistaciifolia Kunth) at three concentrations of soil solution phosphorus. Rev Fac Nal Agro Medellin, 68(1), 7451–7458. doi: 10.15446/rfnam.v68n1.47831. [ Links ]
Siqueira. J.O. & Saggin–Júnior. O.J. (2001). Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza, 11(5), 245–255. doi: 10.1007/s005720100129. [ Links ]
Sousa. N. Franco. A. Oliveira. P. & Castro. M.L. (2009). Ectomycorrhizal fungi as an alternative to the use of chemical fertilizers in nursery production of Pinus pinaster. J Environ Manage, 95(Suppl:S), 269–274. doi: 10.1016/j.jenvman.2010.07.016. [ Links ]
Turner. B.L. Frossard. E. & Oberson. A. (2006). Enhancing phosphorus availability in low fertility soils. En N. Uphoff (Ed.), Biological approaches to sustainable soil systems (pp. 191–205). Boca Raton, United States: CRC Press. [ Links ]
Yuan. L. Huang. J.G. Li. X.L. & Christie. P. (2004). Biological mobilization of potassium from clay minerals by ectomycorrhizal fungi and eucalypt seedling roots. Plant Soil, 262(1), 351–361. doi: 10.1023/B:PLSO.0000037055.67646.97. [ Links ]
Zaidi. A. Khan. M.S. Ahemad. M. Oves. M. & Wani. P.A. (2009). Recent advances in plant growth promotion by phosphate–solubilizing microbs. En: M.S. Khan, A. Zaidi, y J. Musarrat (Eds.), Microbial strategies for crop improvement (pp. 23–50). Berlin, Germany: Springer. [ Links ]
Zangaro. W.V.L. Nisizaki. S.M.A. Domingos. J.C.B. & Nakano. E.M. (2003). Mycorrhizal response and successional status in 80 woody species from south Brazil. J Trop Ecol, 19, 315–324. doi: 10.1017/S0266467403003341. [ Links ]