Introduction
In the generation of scientific knowledge and research, as fundamental elements to assure biodiversity conservation (Josse C. et al., 2009), the José Celestino Mutis Botanical Garden of Bogotá (JBB) employs plant tissue culture as an ex situ conservation strategy of promising native high Andean and paramo species. Two prioritized species within this line of research are Rubus macrocarpus Benth. and Rubus bogotensis Kunth (Rosaceae). The distribution of the giant blackberry and the granizo blackberry, common names used for these two species, is found among 2100 and 3200 m.a.s.l. like other species of the genus Rubus, they have potential nutritional, medicinal, industrial, or ecological uses (Guzmán-Castañeda et al., 2007) and, due to their wild condition, they could be useful for the improvement of economically important commercial crops. Andrade, Córdoba, Criollo, & Lagos (2013), mention that since wild species could be a source of genetic variability, they represent an alternative for solving problems related to plant sanitation, based on their desirable characteristics such as pest and disease resistance. Apart from being promising species, R. macrocarpus and R. bogotensis show propagation difficulties through traditional methods, mainly from seed. The germination process is very slow (beginning two or three months after planting) and occurs at very low percentages, especially for R. macrocarpus (1%) (Córdoba, Guzmán-Castañeda, Pérez-Martínez, Zúñiga-Upegui, & Pacheco, 2010). Owing to these limitations, the development and adaptation of in vitro propagation techniques is proposed as an alternative for ex situ blackberry conservation. This research aimed to evaluate methodologies for in vitro multiplication and rooting (from vitroplants available in the laboratory) and for the ex vitro adaptation (acclimation and greenhouse) of the plant material generated under laboratory conditions.
Materials and methods
This research was carried out in the laboratory of plant tissue culture of the Scientific Subdirection at the JBB. The starting materials were in vitro Rubus macrocarpus and Rubus bogotensis seedlings obtained from in vitro seed planting and germination. The following describes the phases developed for this in vitro plant material:
Multiplication phase
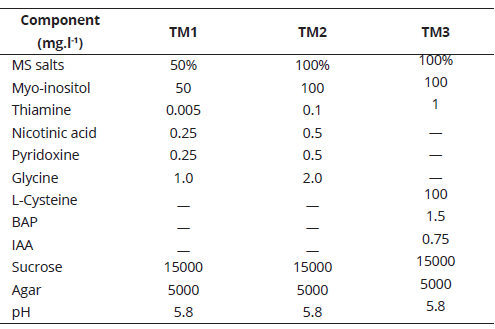
Seedlings with an adequate general development, related mainly with vigor, height, and color, were selected. The leaves and necrotic tissue were eliminated; individual shoots, with an average length of 0.4 cm to 0.5 cm, and one to two axillary buds were cut and planted in the treatments described in Table 1.
Culture media composition was based on mineral salts, vitamins, and myo-inositol in MS medium (Murashige & Skoog, 1962) at 50% (50% MS) or 100% (MS), with or without supplementation (L-Cysteine, BAP or IAA). Apical length (cm) and shoot number were evaluated every seven days, during eight weeks. Increases in these variables were determined based on the average initial and final measurements. The experimental unit was composed of one explant in a culture flask. For R. macrocarpus, 15 repetitions per treatment were used, for a total of 45 weekly measurements for each variable. For R. bogotensis, 12 repetitions per treatment were used (36 weekly measurements for each variable).
Rooting phase
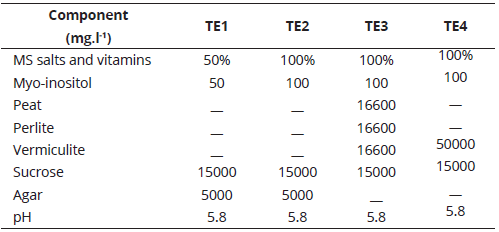
The shoots or stem cuttings generated in the previous phase were transferred to four treatments (Table 2).
After eight weeks, the seedlings were removed from the flasks and the number of rooted shoots and roots were counted. The experimental unit corresponded to one seedling in a culture flask. For R. macrocarpus, 15 repetitions per treatment were evaluated, for a total of 60 plants. For R. bogotensis, a total of 48 plants were managed (12 repetitions per treatment). For elaborating the multiplication and rooting phase media, the nutrients were weighed on an analytical precision balance and dissolved in micro-filtered water; glass containers with 100 ml-capacity were used to distribute the treatments; pH was adjusted to 5.8 and sterilization was carried out in an autoclave at 15 pound-force per square inch (15 lb.in-2) for 15 minutes, with an approximate steam temperature of 121.5°C. For preparing rooting media TE3 and TE4, 50000 mg of substrate were placed into sterilized glass containers, followed by 30 ml of MS with 100% salts, vitamins, and myo-inositol mix (pH adjusted). The explants were kept in the incubation room, under a natural photoperiod (12.12-1), with a temperature range between 19ºC and 27°C, and relative humidity of 60% to 80%. Light intensity was between 1500 lux and 5000 lux.
Ex vitro adaptation phase
Two subphases related to acclimation and greenhouse growth were evaluated. The following describes the methodology applied for each one:
Acclimation. The in vitro plants, with caulinar, foliar, and rizogenic development, from MS medium with 50% salts, vitamins, and myo-inositol (50% MS), were transplanted to pots with a mix of soil, rice husks, peat, and perlite substrate in equal proportions. The pots were located in pans with a transparent plastic cover, with relative humidity controlled. The covers were gradually lifted since day 30 after the transplant and fully removed two months later. Irrigation, by capillarity, was managed twice a week. Twenty four seedlings from each species were randomly chosen in order to measure survivorship (%) and stem growth length (cm) every three weeks. The total evaluation time was three months, during which five measurements were taken. The corresponding increases were determined based on the average initial and final stem growth length (cm) values.
Greenhouse. The plant material was transplanted into black plastic bags containing the same acclimation substrate (soil, rice husks, peat, and perlite mix). A vertical tutor was placed for each seedling in order to provide support. The bags were transferred to the greenhouse, where daily irrigation through aspersion and foliar and edaphic fertilization was managed every five weeks. Survivorship (%) and stem growth length (cm) were evaluated during four months. The first evaluation was done two weeks after greenhouse establishment. Subsequently, measurements were made every 15 days, for a total of eight data recordings for 75 R. macrocarpus and 45 R. bogotensis seedlings. The increase in seedling height was determined based on the initial and final stem length (cm) values measured in each subphase.
Statistical analysis
A completely randomized experimental design was used for each of the phases developed. For data analysis, an analysis of variance (ANOVA) was used and a test for difference of means was calculated through Duncan's multiple range test (p< 0.05), using the statistical software SAS(r) 9.0. A descriptive analysis was carried out for apical height increase and shoot number in the multiplication and ex vitro adaptation phases.
Results and discussion
Multiplication phase
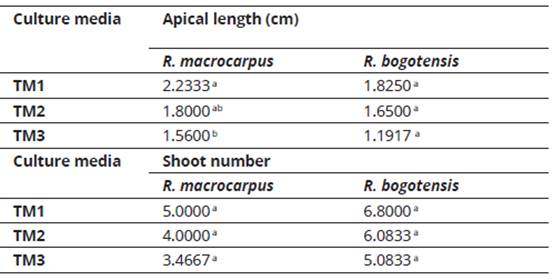
The results for apical length (cm) and shoot number in the last week evaluated allowed to establish that apical length (cm) for R. macrocarpus was the only variable which showed significantly differences (P= 0.026) among culture media treatments, where TM1 culture media (50% MS) showed the highest mean value (2.23). The effect of culture media on the studied variables for both blackberry species is shown in Table 3.
Table 3 Effect of culture media on multiplication phase of R. macrocarpus and R. bogotensis (56 days after planting).
* Means with different letters in the same column differ at p<0.05 (Duncan notation)
Despite the ANOVA results, differences in culture media, related especially with vigor, were observed after finalizing the evaluation. Seedlings from TM3 (MS + thiamine 1 mg.l-1 + L-cysteine 100 mg.l-1 + myo-inositol 100 mg.l-1 + BAP 1.5 mg.l-1 + IAA 0.75 mg.l-1), did not develop satisfactorily, some of them presented oxidation and withering. The opposite was observed for seedlings planted in the other treatments, mainly in TM1 (50% MS), where large and bright leaves and firm and vigorous stems were observed.
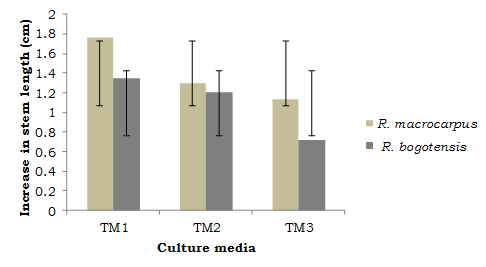
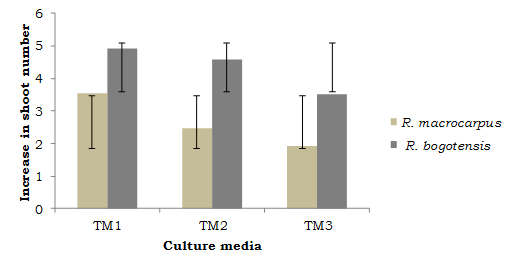
These observations relate to the descriptive analysis regarding increase in the studied variables (comparison between average initial and final values). As can be seen from Figures 1 and 2, R. macrocarpus and R. bogotensis explants in medium TM1 reached the highest increase values for apical length (cm) and shoot number.
Considering the combined use of phytohormones (6 BAP + GA3; 6 BAP + IAA or BAP + GA3) for species of the genus Rubus, such as Rubus glaucus Benth., is appropriate for achieving the greatest length and shoot number in explants (Sigarroa-Rieche & García-Delgado, 2011; Marulanda, Carvajalino, & Vento, 2000), it can be noted that for the wild species evaluated here, growth regulators are not required to produce complete plants from microcuttings. The latter is associated with a 50% reduced concentration in salts, vitamins and myo-inositol in the MS medium, which was determined to be valid for this phase. The advantage in explant growth and development, which is seen with the use of TM1, is accompanied by a cost reduction in the in vitro production of plant material.
Stem elongation is a key parameter which is strongly affected by culture exposition time (Uribe-Moraga & Cifuentes, 2004). Sigarroa-Rieche & García-Delgado (2011), mention that hormonal combinations of BAP (1.5 mg/l) + IAA (0.75 mg.l-1) and BAP (1.5 mg.l-1) + IBA (0.75 mg.l-1) did not provide satisfactory results in Rubus glaucus Benth., plant length and its multiplication coefficients, in addition to 81% to 84% callus formation. In this research, callogenesis was not observed for R. macrocarpus and R. bogotensis under any of the treatment influence, which is considered to be an advantage, since callus appearance tends to weaken the existing shoots (Marulanda et al., 2000).
Shoot number is an equally important variable in the multiplication phase, since with greater shoot numbers, a greater multiplication phase is obtained (Solis, Olivera, & La Rosa, 2011). Villa et al., 2005 (cited in Sigarroa-Rieche & García-Delgado, 2011), evaluated the in vitro multiplication of blackberry (Rubus sp.) and determined that a greater shoot number (3.99) is obtained in a culture medium with 1 mg.l-1 BAP. This value was greater for R. macrocarpus and R. bogotensis seedlings evaluated in this study, with an average of 5.0 and 6.8 shoots per explant in a salts and vitamins-reduced medium without phytoregulators (TM1). López, (1998), cited in Uribe-Moraga & Cifuentes, (2004), mentions that, in many species, bud development is favored by the use of salt-reduced culture media.
Rooting phase
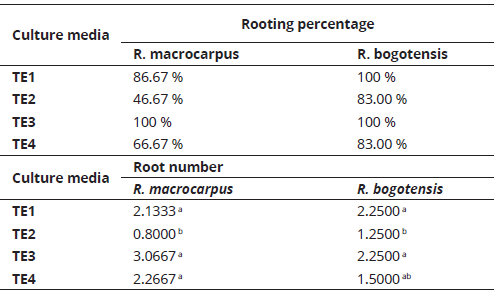
Rooting percentage per treatment was determined 56 days after planting, and prior to the statistical analysis of root number. For R. macrocarpus and R. bogotensis, TE3 provided the best root number percentages (100%). For R. bogotensis, TE1 also provided 100% rooting. Significant differences were seen for root number between treatments. In R. macrocarpus (P= 0.0062), TE1, TE3, and TE4 media formed a homogenous group, where TE3 showed the highest mean value. For R. bogotensis (P= 0.0271), TE1 and TE3 displayed the highest mean root number values (Table 4).
Accordingly, TE1 (MS al 50%) and TE3 (agar was replaced by inert substrate composed of peat, perlite, and vermiculite) are the most adequate media for obtaining the highest rooting percentages and root number. However, under TE1, plants showed greater vigor, color, and foliar development; thus, it is considered to be the most indicated medium for the in vitro rooting phase.
Salt reduction has been shown to affect root induction (Flores, Chacón, Jiménez, & Ortiz,2012), since it generates physiological stress in plants, which leads to root production in search for potential far away nutrient sources, accelerating radicular development (Salisbury & Ross, 1994). Auxins (NAA, IBA, IAA, and 2-4 D) induce in vitro root formation (Pedroza-Manrique & Bejarano-Tibocha, 2008). In Flores et al., (2012), 50% MS medium, supplemented with 0.5 mg.l-1 IBA, achieved the greatest root length averages in Rubus adenotrichus Schltdl. shoots. However, Vaca & Landázuri (2013), and Alev & Arslan (2006), throughout their studies in roses, determined that MS medium reduced 50% in salts and vitamins or nitrates, without hormonal supplements, favored root number. These results, in which no phytoregulators were used for in vitro rooting, agree with those obtained in this study for R. macrocarpus and R. bogotensis.
Although, with the use of inert substrate instead of agar (TE3) similar effects were obtained in rooting percentage and root formation, it was unfavorable for plant vigor, probably because this medium contained 100% salts, vitamins, and myo-inositol from the MS medium. Nevertheless, an inert substrate, together with 50% salts, vitamins, and myo-inositol from MS, could be a cost reducing alternative that can also be used in the shoot multiplication phase, according to Martínez-Hernández, Alonso, Osorio, Gallardo, López, & Mata, M. (2009). The use of simple culture media is related with greater current knowledge regarding the other factors that affect in vitro culture (Pedroza-Manrique & Bejarano-Tibocha, 2008), and is determined by endogenous auxin and cytokine concentrations present in the explants, which depend on the species and the explant type (Pedroza & Micán, 2006). In this manner, in vitro cellular processes are favored by synergic concentrations between endogenous and exogenous phytohormones (Salisbury & Ross, 1994).
Ex vitro adaptation phase
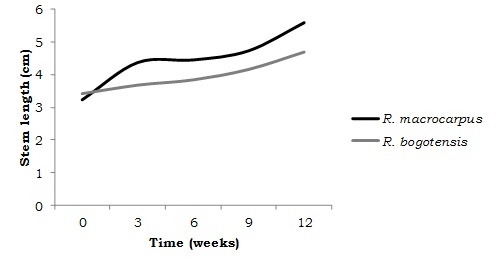
Acclimation. Acclimation is one of the most care-requiring phases in in vitro propagation, therefore, environmental factors, such as relative humidity, light intensity (Martínez-Montiel et al., 2011), irrigation frequency, and substrate quality (Marulanda et al., 2000) must be controlled. In this study, relative humidity in the transparent-covered pans was maintained around 90% and the average daytime temperature for acclimation was 24°C. Under these conditions and after three months of acclimation, survival percentages for R. macrocarpus and R. bogotensis were 83.33% and 75%, respectively. With the substrate and environmental conditions described, increases of 2.35 cm and 1.30 cm in stem length were obtained in 12 weeks of evaluation (Figure 3).
The survivorship results found in this study support those reported by Flores et al., (2011), for R. adenotrichus, where the maximum survival achieved in four weeks of acclimation was 78.6% for plants grown in MS medium with 50% salts and supplemented with 0.250 mg.l-1 IAA.
Seedling death evaluated in this study was mainly due to dehydration by water stress and, in lesser proportion, to the presence of Penicillium nalgiovense (non-pathogen, environmental fungus) in the leaves and stems of the evaluated plant material, which appeared between the third and sixth weeks after transplant. Vitroplants, compared to plants produced in growth chambers or greenhouses, are fragile and vulnerable to environmental conditions, since they are morphologically, anatomically, and physiologically abnormal (Pospíšilová, Tichá, Kadleček, Haisel, & Plzáková, 1999). They have a low epicuticular wax content (Martínez-Montiel et al., 2011), and low stomatal function and photosynthetic activity, due to which the acclimation period is necessary for correcting these abnormalities (Nava et al., 2011).
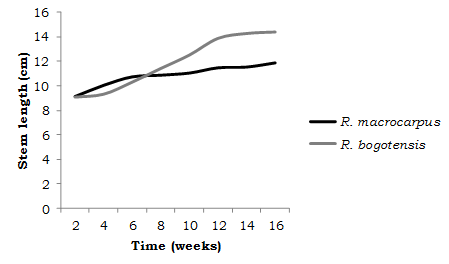
Greenhouse. The environmental conditions under which this subphase was carried out into an average daytime temperature of 20°C and nocturnal temperature of 12°C. Average environmental humidity was 65% (diurnal) and 85% (nocturnal). For the 75 R. macrocarpus and 45 R. bogotensis seedlings, survivorship was 100% after four months of greenhouse follow-up. The latter indicates that the acclimation phase was adequately managed, since it allowed the formation of vigorous plants that displayed high survival levels when transplanted and transferred to the covered system. Based on the average initial and final stem lengths (cm), there was an increase of 2.75 cm for R.macrocarpus and 5.31 cm for R. bogotensis (Figure 4).
Conclusion
For in vitro multiplication and rooting phases, the best culture medium was MS at 50% salts, vitamins, and myo-inositol, supplemented with 15000 mg.l-1 sucrose and 5000 mg.l-1 agar. Under this culture medium, endogenous seedling concentrations could autoregulate, favoring caulinar, foliar, and rizogenic formation. In ex vitro adaptation, the acclimation and greenhouse subphases were overcome by using plants from 50% MS medium, transplanted in a soil, rice husks, peat, and perlite (1:1:1:1) substrate. The substrate employed and the environmental conditions managed allowed for acceptable survival levels and seedling height.
Considering that there are few reported studies on wild Rubus species, the methodology presented here constitutes a basis for developing micropropagation processes of species selected under criteria related mainly with degree of threat, difficulty of propagation under traditional methods, population accessibility, plant material availability, and potential for sustainable use