Introduction
The cherimoya (Annona cherimola Mill.), has its center of origin in the southern part of Ecuador, northern Peru and Bolivia (Van Zonneveld et al., 2012). This fruit is widely appreciated for its taste, aroma and nutrient content. In Ecuador, this crop is mainly located in the provinces of Loja, Azuay, Pichincha, Imbabura and Tungurahua, in the Inter-Andean Region of Ecuador, at altitudes ranging from 1500 to 2600 m. a. s. l. According to the National Institute of Statistics and Census of Ecuador, the country has a total of 16201 trees of this fruit in the whole country, with a production of 447 t, which is low compared to the great demand for consumption of this fruit.
Most species of fruit trees require a period of growth in the nursery prior to transplantation in commercial orchards. In this stage, the use of mycorrhizal-arbuscular fungi show great potential because they play an important role in the growth and nutrition of higher plants, such as fruit species (Vázquez et al., 2011).
These fungi are obligate symbionts and need to be associated with the root of their hosts to obtain carbon from the photosynthesis. In exchange they return to the plant the nutrients and water that are extracted from the soil. In addition, they represent a prolongation of the roots of the plant beyond the nutrient depletion zone (Saravesi et al., 2014). According to the above, mycorrhizal fungi facilitate the development of cherimoya seedlings due to increased absorption of nutrients, mainly phosphorus, an element responsible for the development of new cells and thus plant growth.
The benefits of inoculation with fungi that form arbuscular mycorrhizal are expressed in enhanced seedling survival; increased plant growth in less time, thus reducing time spent in the nursery, savings in fertilizer costs; and increases in production and product quality (Verbruggen et al., 2013). The modification of the root system by symbiotic association with mycorrhizal fungi helps to improve the absorption and transport of water and nutrients from the soil to the root through the increase in the volume of soil explored, which is reflected in the improvement in plant growth (Verbruggen et al., 2013).
In agriculture, mycorrhizal fungi deserve special interest for their effect on plant growth through increasing the efficiency of the root system in the absorption of nutrients, especially phosphorus, and promoting absorption of trace elements (Smith et al., 2015). Furthermore, the use of these fungi reduce the application of pesticides because mycorrhizal create physical barriers or activate defense mechanisms against root pathogenic organisms associate with various metabolic routes (Goicoechea et al., 2013), thus becoming an environmentally friendly alternative that helps to preserve the environment and promotes an approach to new technologies orientated towards sustainable management that can be included in the process of integrated fruit production (Verbruggen et al., 2013). According to this background. The aim of this research was to evaluate the effect of mycorrhizal fungi on the growth promotion and development of seedlings of cherimoya cultivar 'Cangahua' in the nursery.
Materials and methods
Study area
The research was conducted in the laboratory (23 °C and 41% relative humidity) and in the greenhouse (29 °C and 37% relative humidity) of the Tumbaco Experimental Farm belonging to the National Institute of Agricultural Research, located at Tumbaco in the Province of Pichincha, County of Quito, at an altitude of 2348 m. a. s. l., latitude 00° 13' 00'' South and longitude 78° 24' 00'' West.
Mycorrhizal content analysis of the soil from cherimoya orchards
Collection of biological material
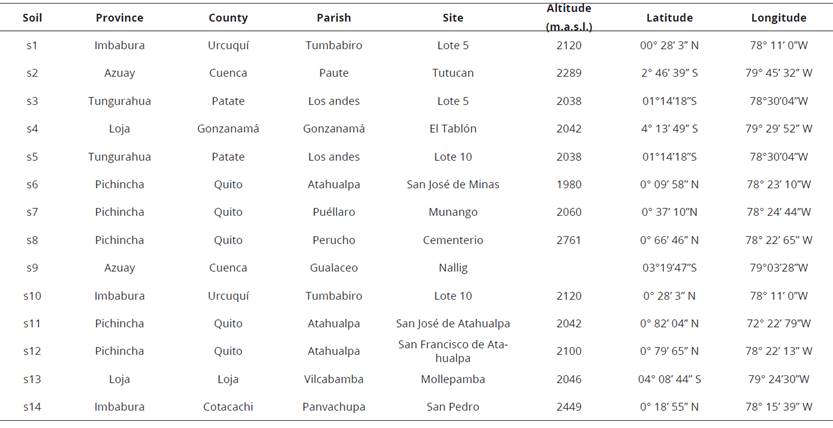
Roots and soil samples were taken in cherimoya producing areas in the Highlands of Ecuador (Table 1).
Soil sampling
To determine the number of spores in soil, sampling was carried out around the crown (dripping zone) of cherimoya plants, selected at random, at a depth of 20 cm. The sample was composed of 10 sub-samples of 100 g each one. They were transported using a cooler to the laboratory and they were dried at environmental temperature (22 oC), under shade, for 15 days.
Root sampling
This sampling was carried out to estimate the root colonization. The treetop extension was used as a reference for taking root samples. A shovel was used to dig to a depth of 40 cm around the tree at the dripping zone in order to obtain secondary and tertiary, healthy, young and slightly lignified roots. Samples were transported using a cooler to the laboratory.
Estimated variables
Mycorrhizal colonization rate in roots: The clarification and root staining method, mycorrhizal spore population in soil: spore quantification was carried out following the described method of Phillips & Hayman, (1970).
Data analysis
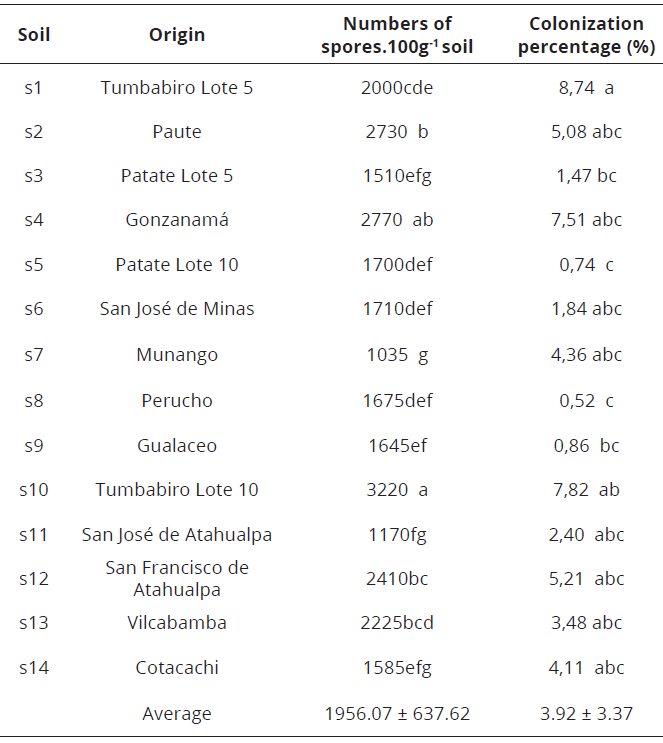
An analysis of variance was carried out. In addition, the Tukey statistical test at 5% to determine significance ranges. The statistical analysis was established in R 3.3.1. sotware (tm). Soils (Tumbabiro-Lote 10, San Francisco de Atahualpa, Paute & Gonzanamá), which showed the best results in number of spores and root colonization (Table 2), were selected to continue the assay in the next phase.
Propagation in trap plants ( Sorghum vulgare )
Soil sampling for physical-chemical analysis
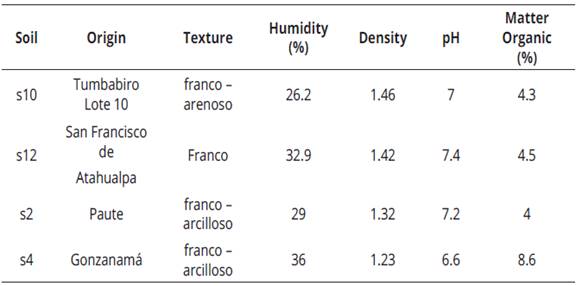
Following the sample procedure described previously, 1 k of soil sample from the four selected soils for evaluation in the trap plants was collected, labeled and sent to the laboratory for examination.
Treatments
Soils that achieved the best averages in spore count and high root colonization, constituted the inoculum that was propagated in trap plants of sorghum variety 'INIAP 201', the treatments being as follow: s1= Tumbabiro, s2= San Francisco Atahualpa, s3= Paute, s4= Gonzanamá and s5= control (sterile sand).
Experimental design and statistical analysis
A randomized complete block design with three replications was used. In addition, Tukey test at 5% was done to determine differences between treatments. The experimental unit consisted of a pot of 2 k of capacity with 500 g of sterile sand placed in the bottom, then 1 kg of soil (treatment) and a layer of sterile sand (100 g). Ten seeds of sorghum were sown in each pot. The statistical analysis was carried out in R software version 3.3.1. (tm).
Estimated variables
Total phosphorus concentration in plant tissue: After 3 months, total phosphorus concentration in plant tissue was determined by chemical composition analysis. Percentage of dry matter produced: After 90 days, the fresh weight of root, stem and leaves was measured. Samples were placed in an oven for 24 hours at 110 °C to determine the dry weight and establish their percentage ratio. Population of mycorrhizal spores in the soil: Quantification of spores was carried out 90 days after the seeding of the trap plants, using the sieving and centrifugation method (Phillips & Hayman, 1970). Mycorrhizal colonization rate in roots: This was evaluated 90 days after the seeding of trap plants, using the method of clarification and staining roots, following the technique of Phillips & Hayman (1970), together with the method of visual density.
Specific management of the experiment in trap plants
Sorghum seeds were placed in rubbing alcohol for 3 minutes, washed with a solution of chlorine at 1.5% for 2 minutes and rinsed five times with sterile distilled water. In each pot was placed 500 g of sterile sand (water vapor at 80 oC for 1 hour), 1 kg of soil sampled and finally a layer (100 g) of sterile sand, and ten seeds were planted. At 90 days after planting, three plants at random were taken and root colonization was assessed. Using 20 g of the sieved soil, spores determination by sieving and centrifugation method was performed. Three other plants were used for determining the percentage of dry matter. The four remaining plants were maintained to continue generating the inoculum that was used in the nursery stage. Ten days before using the inoculum, the trap plants were cut by the stem base and watering was stopped. The inoculum was prepared by mixing the roots of the trap plants, which were cut into pieces of about 1 cm, with the potting soil.
Evaluation in cherimoya seeds and seedlings
Once propagated the inoculum of each of the four selected soils was assessed in nursery conditions. This phase was divided into two trials where the inoculum was evaluated in cherimoya (cultivar 'Cangahua') seeds and seedlings.
Treatments
These were constituted by four inoculums, a commercial control and an absolute control: i1= Tumbabiro, i2= San Francisco de Atahualpa, i3= Paute, i4= Gonzanamá, i5= commercial inoculum; i6= control (black soil, pomina and rice husk, all sterilized).
Evaluated variables
Rate of mycorrhizal colonization in roots: At the fourth month after the inoculation of the cherimoya seeds and seedlings (3 months old), the rate of colonization was determined by the technique of Phillips and Hayman (1970). Dry matter percentage: At 120 days after inoculation of seeds and seedlings, the fresh weight of root, stem and leaves was measured in plants, and then samples were placed in an oven for 24 hours at 110 ° C to measure the dry weight and calculate the percentage of dry matter. Total phosphorus: After 90 days, the total phosphorus was estimated by foliar analysis using the molybdenum-vanadate colorimetric method, while the molybdenum blue method was applied for phosphorus determination in the soil (AOAC, 1995).
Experimental design and statistical analysis
A randomized complete block with five replications was performed. In addition, Tukey test at 5% was estimated to determine differences among the means of treatments. The experimental unit consisted of a seedling cherimoya planted in a bag containing 2 kg of substrate with 100 g of inoculum. The statistical analysis was established in R software version 3.3.1. (tm).
Specific management of cherimoya seeds and seedlings
Seed disinfection: Seeds of cultivar 'Cangahua' were placed in rubbing alcohol for 3 minutes, then were soaked with a solution of chlorine at 1.5% for 2 minutes and rinsed five times with sterile distilled water. Seed pre-germination: Seeds were immersed in a solution of gibberellic acid at a concentration of 1250 ppm for 24 hours. Sowing: In plastic bags (2 k of capacity), 1.5 k of sterile substrate (black soil, pomina and rice husk in proportion 2: 1: 1) and 100 g of the inoculum was placed in each treatment. The substrate was sterilized using water vapor at 80 oC for 1 hour. One seed of cherimoya was sown and covered by 100 g more of the sterile substrate. The seedlings were previously germinated and after 90 days of growing were transplanted into the bags. Fertilization: This was done using ammonium nitrate at a dose of 2 g.l-1 of water.
Results and discussion
Mycorrhizal content analysis of the soil
According to the results, there is a moderately acceptable correlation (0.64) between the number of spores and the percentage of colonization. Soils that showed the highest values in these two variables corresponded to samples collected in Tumbabiro-Lote 10 (Imbabura), Gonzanamá (Loja), Paute (Azuay) and San Francisco de Atahualpa (Pichincha) (Table 2), being in these soils the genus Glomus as the most frequent. The best results were recorded in the samples from Tumbabiro with a sandy loam texture (Table 3), which allows good drainage, as mentioned also by López et al. (2015), who stated that there is a greater presence of these microorganisms in well-drained soils. Moreover, mycorrhizal fungi act in the soil structure by providing more aggregation and stability, and preventing erosion processes (Lozano et al., 2015).
Propagation in trap plants
Dry matter percentage
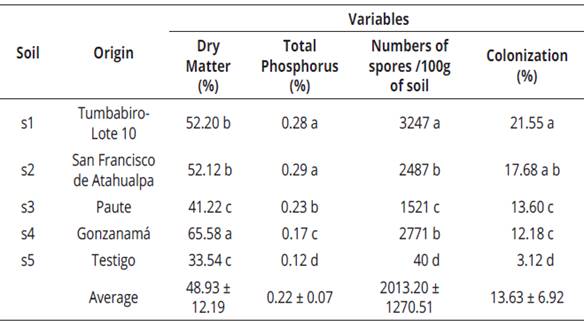
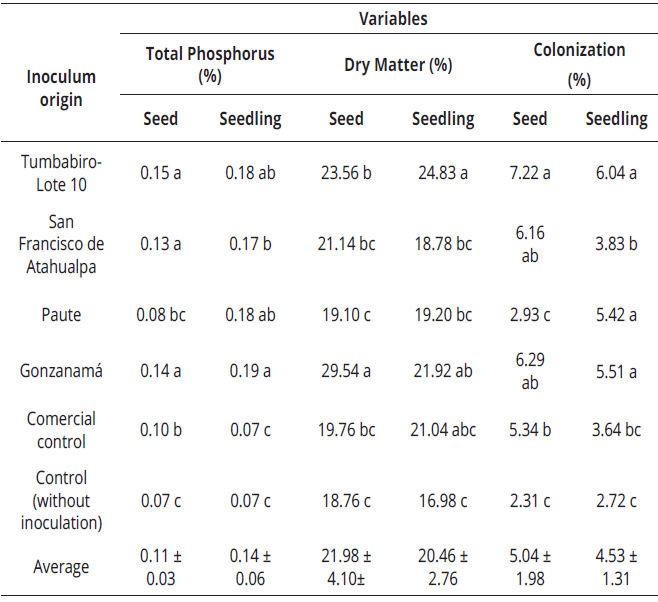
When analyzing this variable (Table 4), it can be shown mycorrhizal treatments showed the best results, consistent with those reported by Bressan et al. (2001), who found mycorrhizal fungi contribute to increase dry matter. The inoculum produced with the soil from Gonzanamá obtained the highest value, with an average percentage of 65.58% of biomass, whereas the control without inoculation obtained 33.54%. Díaz et al. (2014), mention that in S. vulgare, it has been observed that mycorrhizal plants have increased growth, photosynthetic pigments and stomatal conductance compared with plants without mycorrhizal inoculation. Apparently the carbohydrate flow is regulated by the host plant and depends on the species of fungus.
Total phosphorus
The highest percentage of phosphorus was observed in plants inoculated with soil from Tumbabiro and San Francisco de Atahualpa with an average rate of 0.28% and 0.29%, respectively; while the control (without inoculation) obtained a much lower value (0.12%) (Table 4). Smith et al. (2015), reported that mycorrhizal fungi contribute to greater absorption of N, P, K, Zn and Cu by the plant, a result also confirmed by the research done by Díaz et al., (2014).
Mycorrhizal spores population in soil
The largest population of spores was observed in the treatment with the soil from Tumbabiro with an average of 3 247 spores.100 g-1 of soil, while the lowest percentage was found in the control with 40 spores.100g-1 of soil (Table 4). Pérez (2011), mentions that compacted soils reduce soil fertility and distribution of plant roots and, therefore, the hyphae of arbuscular-mycorrhizal fungus in the rhizosphere decrease. In addition, the formation and function of mycorrhizal fungus may be affected, finding the soil environment can favor the development of fungi at one time and reduce it at another either by direct effects on fungal communities or indirectly through the effect of the soil environment on the host plant.
Rate of mycorrhizal colonization in roots
The highest percentage of root colonization was observed in the treatment with the soil from Tumbabiro, with an average of 21.55%; while the lowest percentage (3.12%) was shown by the control (Table 4). According to these results, it is deduced that the kind of spores of the Tumbabiro soil were effective in the trap plants evaluated because the infection rate exceeded 20%, being a positive result as mentioned by Herrera (1993), which it is important because the inoculum is from the original soil without increment of the population prior to inoculation. Colonization in control plants occurred due to the capacity of survival of mycorrhizal spores in the soil, although the substrate was disinfected.
Evaluation in cherimoya seeds and seedlings
Dry matter percentage
As in the previous case, no statistical difference (p-value = 0.32) was found between seed and seedling inoculation. Seedlings with inoculum obtained from soils of Tumbabiro and Gonzanamá showed the highest percentages of dry matter compared to the other treatments (Table 5). This benefit could be explained by the colonization and action of mycorrhizal fungi. Posada & Franco (2006), reported the interaction of rhizosphere microorganisms with mycorrhizal fungi, which allows the symbiotic association to produce a greater effect on colonization, and therefore in the production of dry matter since absorption and nutrient availability is greater for the plant. Furthermore Saravesi et al. (2014), report the synthesized carbohydrates in leaves, have allowed the development of mycorrhizal fungi. These carbohydrates are more developed in inoculated seedlings than in seeds because seedlings start with a certain amount of leaves and are capable of carrying out photosynthesis, benefiting the fungus with carbohydrates.
Total phosphorus
There was no statistical difference (p-value = 0.14) when the treatment means were analyzed jointly in the percentages obtained in seed and seedling inoculation. Independently, both seeds and seedlings showed statistical differences among treatments. The largest proportion of absorbed total phosphorus was observed in the seedlings planted with the inoculum from Tumbabiro & Gonzanamá (Table 5), implying that this occurred due to the action of the mycorrhizal fungus that perform their function through external hyphae that increase the number of absorption sites (Noda, 2009). In addition, Meinhardt & Gehring (2012), found there is a greater absorption of phosphorus because mycorrhizal fungi change the pH of the soil by organic agents and solubilizers such as phosphatase, making available phosphorus which is retained in the soil, as occurred in our study (Table 6).
Table 6 Percentage of phosphorus retained in the soil before and after mycorrhizal inoculation in cherimoya seeds and seedlings
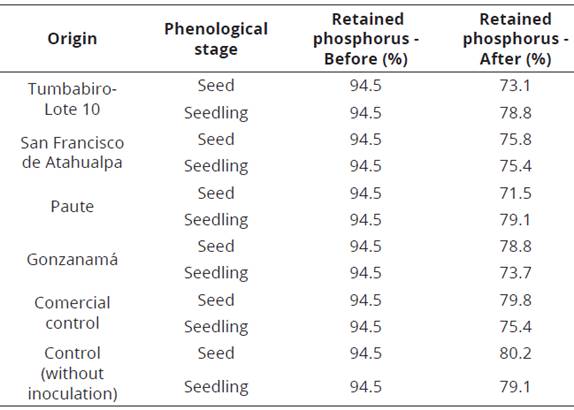
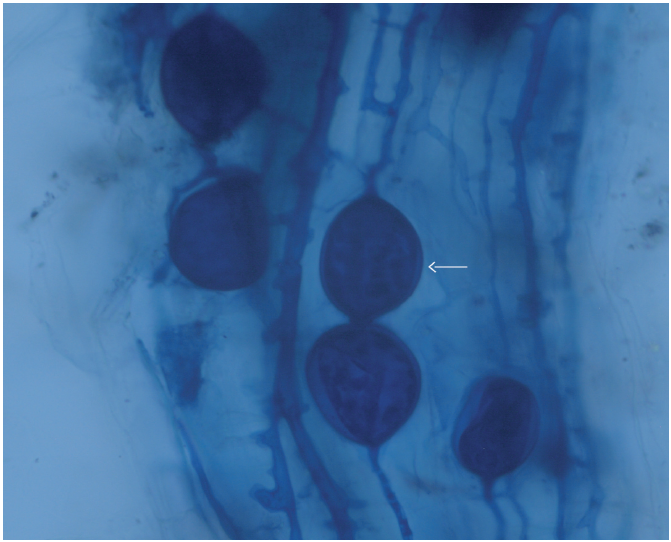
Studies have shown the phosphorus concentration in plant tissue is related to the structure that colonizes the root, namely, when the root is colonized by arbuscules, the amount of phosphorus in tissue decreases because fungi also absorb phosphorus for growth; whereas phosphorus becomes available to the plant when the root is colonized by vesicles and mycelium as occurred in this research (Figure 1), and often vesicles act as a reservoir (Vanhove & Van Damme, 2013).
Rate of mycorrhizal colonization in roots
There was no statistically significant difference (p-value = 0.5) in colonization rate when comparing jointly the treatment means of the inoculation in seeds and seedlings. This is consistent with those results reported by Carreón et al. (2014), who stated the inoculation of these fungi can be performed in seedbeds or in transplantation. Inoculation in seedbeds could present certain problems because seeds can germinate slowly or, when they germinate, may produce certain allelopathic compounds that inhibit the process of intra-radical colonization by mycorrhizal fungi. Moreover, in most tree fruit species, seedlings depend on the reserve content of their cotyledon leaves, thus inoculation might be needless (Carreón et al., 2014).
In seedling, the highest percentage of colonization (Table 5), was obtained by the treatment in which the inoculum from Tumbabiro (7.22%) was used. This percentage is apparently low because of the short time of assessment (4 months) between inoculation and the colonization analysis, taking into account that the initial growth of the root and vegetative development are slow. Control showed a percentage of colonization of 2.72%, it can be explained by the persistence of spores in the soil due to populations of mycorrhizal fungi are composed of spores of different ages and in distinct states of dormancy or quiescence (Montañez et al., 2010). Studies show in perennial species more time is required to obtain higher and steady colonization percentages because fruit crops such as cherimoya have an extended life cycle which can reach more than 30 years of life (Posada & Franco, 2006).
Conclusion
Soils sampled in Tumbabiro and Gonzanamá (main cherimoya producing areas) showed the highest populations of mycorrhizal spores and colonization rates in cherimoya roots. There was a 100% increase in the absorption of phosphorus compared to the control (without inoculation) in the trap plants where these soils were used as inoculum. Cherimoya seedlings inoculated with inoculum made with soil from Tumbabiro showed the highest colonization of roots, dry matter and accumulated phosphorus in the plant tissue. These results suggest that the use of mycorrhizal fungus is an alternative to improve the nutrient absorption, mainly phosphorus, and increase dry matter, which is reflected in the seedling development.