Introduction
Guarianthe skinneri (Bateman) Dressler & W.E. Higgins., is distributed from Mexico to Panama and is commonly known as "La Candelaria" (Damon & Bertolini, 2015). In Mexico, is in the state of Chiapas, where it historically had a wide distribution, now restricted to the Biosphere Reserves El Triunfo and Tacaná Volcano. Actually, G. skinneri, is in the category of threatened in the Official Mexican Norm "NOM-ECOL-SEMARNAT-059-2010", due to the Unsustainable extraction and reduction of their natural habitat. In addition, as all orchid seeds, lack endosperm and cotyledons, so they contain few nutrients or reserves to perform in vitro germination. However, in natural conditions must be associated with mycorrhizal fungi, which allows to plant development (Bertolini et al., 2014; López-Chávez et al., 2016).
To massively propagate G. skinneri, it is possible to resort a clonal propagation from in vitro culture techniques, but at present, there are no an adequate propagation protocols for this orchid. This alternative would allow the specific germplasm preservation and outstanding genotypes. This would cover the local demands of this species as an ornament plant without disturbing the wild populations. However, an in vitro culture technique common limitation is the explants contamination, which have allowed the report from 13 to 100% in vitro seeds and tissues contamination collected from field conditions (Chávez et al., 2015; Soetopo & Lestari, 2012).
To date, there have been no reports of in vitro orchid culture using pseudobulb pith explants. The only reports of in vitro propagation of G. skinneri, correspond to the research of Coello et al. (2010). Explants of lateral vegetative apices were used in MS medium (Knudson, 1946), with the combination of NAA (Naphthalene acetic acid) and Kin (Kinetin) plant growth regulators. It was obtained 20% survival of explants due to contamination and oxidation, from which 66.67%, induced protocorms formation. Coello et al. (2010), evaluated the GA (gibberellic acid), BAP (Bencil amino purine), NAA and IA (Indoleacetic acid) combination with plant growth regulators in MS medium for in vitro germination of G. skinneri, obtaining a higher number of new shoots (10.2) and roots (3.7) per plant with the combination of NAA at 16.1 μM, IA at 17.1 μM and BAP at 4.9 μM.
Clonal propagation has been reported for several types of orchid, the most widely propagated in vitro at the world level are Phalaenopsis, Dendrobium, Laelia, among others, due to its wide demand in the ornamental market (INEGI, 2016). In these species, the explants most used are leaves, buds, seeds and seedlings, which have allowed the induction and in vitro germination of callus, leaves and shoots as well as seed germination. Based on the above, it is necessary the identification of the type of explant, which propitiates low percentages of contamination, as well as the plant growth regulators, which have allowed the in vitro callogenic induction of G. skinneri as a first step to clone, for example, selected individuals for its aesthetic characteristics. Therefore, the aim of this research was to induce in vitro callogenesis in explants of Guarianthe skinneri by means of plant phytoregulators.
Materials and methods
Plant material collection
Leaf samples and pseudobulbs were collected from 30 G. skinneri plants from different households in the city of Tapachula, Chiapas, Mexico. The municipal territory is located between the parallels 14 ° 37 'and 15 ° 14' north latitude and the meridians 92 ° 09 'and 92 ° 28', with an altitude between 0 and 2700 m. a. s. l. The temperature annually fluctuates between 14 - 30 ° C, and rainfall between 1000 - 5000 mm. The weather is preponderantly humid, warm type with abundant rainfall in summer and warm sub-humid with rainfall in summer (INEGI, 2016).
Tissue explants were extracted from adult and apparently healthy plants, which had two to three pseudobulbs with roots and leaves. For leaf explant, a cutting was established from the base, while for the pseudobulb, a cut was made to remove the roots and floral scape. The samples were placed in plastic bags sprinkled with water and immediately transferred to the Laboratory of Biotechnology of the Rosario Izapa Experimental Station - National Institute of Agricultural and Livestock Forestry Research, located in the municipality of Tuxtla Chico, Chiapas.
Disinfection of plant material
The plant material (leaves and pseudobulbs) washed with tap water and liquid commercial soap, and then a cleaning carried out with cotton swab moistened with 95% of ethyl alcohol. Disinfection carried out under aseptic conditions in laminar flow hood, by soaking each leaf and pseudobulb in 10% (v / v) NaClO solution with one drop of 80% Tween detergent for 10 min.
Three washes were performed with sterile distilled water and left in an antioxidant solution (0.1 g L-1 ascorbic acid, 0.15 g L-1 citric acid and 30 g L-1 of saccharose), until the cuts were made to obtain the explants. In total, 250 leaf explants were obtained, consisting of 5mm2 fragments taken from the apical medial part, as well as 250 pseudobulb pith explants which were fragments of 5 mm in diameter by 5 mm in length, obtained with the aid of a punch (Figure 1).
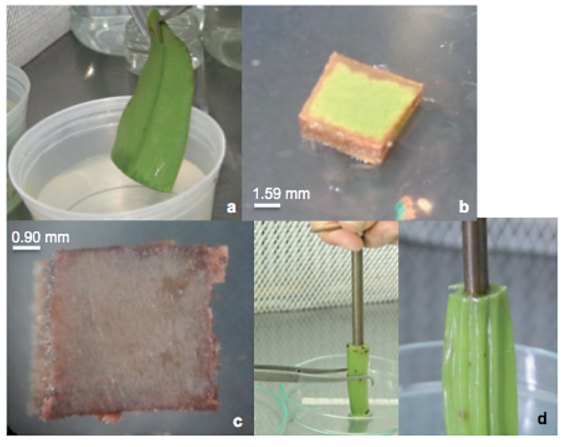
Figure 1 Explants of Guarianthe skinneri. A) Apical leaf part; B) leaf explant at seven days; C and d) obtaining pseudobulb pith.
The explants were sown in 60x15 mm petri dishes, the leaves were established with the beam towards the culture medium and pith explants maintained the polarity at the time of sowing. All explants were maintained at 25 ° ± 2 ° C, illuminated by fluorescent tubes in cold light with a lighting time of 16 h/d, throughout the experimental period.
Callogenesis induction
Callogenic induction was performed in the MS culture medium by adding the growth regulators BAP (Bencylaminopurine), 2, 4-D (2, 4-dichlorophenoxyacetic acid), Kin (Kinetin) and AS (Adenine sulphate) to be evaluated separately and in combination (Table 1). Likewise, a medium without growth regulator, which was considered as a control. An experimental design with two fixed factors performed, which consisted of two-level explant type and five-level growth regulator type. In total, 10 treatments with 50 replicates each were evaluated, generating 500 experimental units.
The variables to be evaluated were as follows: callus formation, oxidation and contamination. Considered as binary variables of presence or absence. Callus formation was observed when undifferentiated cell mass was observed in the explants, oxidation when darkened tissues were observed, and finally contamination by the presence of fungi or bacteria in the medium or in the explant. The evaluated variables from three days after the experiment establishment. Measurements performed every seven days for a period of 49 days.
Data analysis
The data were analyzed using a logistic regression model of repeated measures with binomial response, because the response variables of the experiment were binary type and each experimental unit, measured at different times. We used Statistical Software R version 3.2.2(tm). In addition, orthogonal contrast analysis was performed to know the statistical differences of the evaluated factors by each response variable.
Results and discussion
Seven days after the establishment of the experiment, response of the callus variable was observed in the pith explants. At 49 days, statistical analysis showed the pseudobulb pith generated greater callus formation and presented lower contamination with respect to leaf explants (Table 2).
Always at 49 days, also the callus formation variable was established a statistically significant difference with respect to the growth regulating factor (X2= 40.18, df=4, p-value= 3.45e-15***) and explant type (X2= 3.96, df=1, p-value= 0.04645*). It was demonstrated the growth regulators application to the culture medium, achieved a higher callus formation and greater cell mass formation was observed with the leaf and pseudobulb pith treatments, which were used BAP to 3 mgL-1 (Figure 2, treatments 1 & 6).
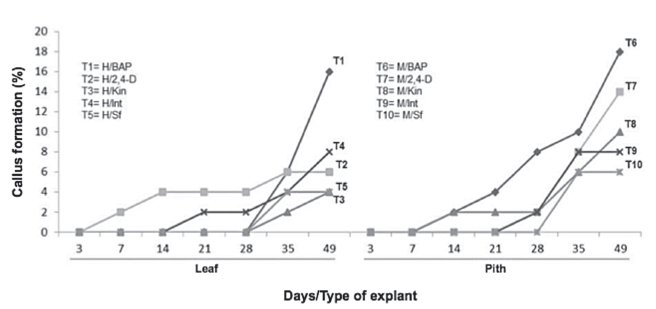
Figure 2 Dynamics of the callus formation percentage in explants of Guarianthe skinneri. H: Leaf explant; M: Pith explant; Int: interaction of growth regulators; Sf: without growth regulators.
In the Pith treatment with BAP (T6), callus production was detected from 14 days, whereas in leaf at 28 days, it can be seen the pith explant with BAP (T6) had a higher response rate than the leaf explant (T1). In pith explants, oxidation was observed from the experiment establishment and it appears as constant during the 49 days (Figure 3).
The explants oxidation of pith was performed in homogeneous way in all the experimental units with five treatments without inhibition of callus formation. In contrast, leaf explants oxidation was observed three days after the experiment establishment, showing significant statistical difference at 14 days (X2= 22.12, df=4, p-value= 0.00018***). Had a higher oxidation percentage with BAP, whereas the treatments with 2,4-D and without growth regulators showed less oxidation (Figure 4).
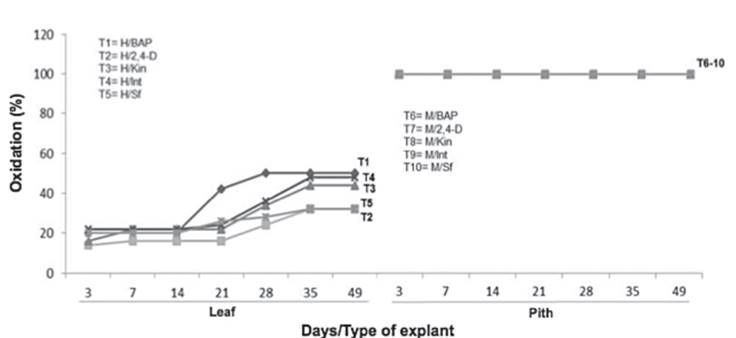
Figure 4 Dynamics of the oxidation percentage in explants of Guarianthe skinneri. H: Leaf explant; M: Pith explant; Int: interaction of growth regulators; Sf: without growth regulators.
The observed contamination always caused by fungi and manifested as early as three days after the establishment. A statistically significant difference was obtained for the evaluated factors for each type of explant (X2= 285.32, df=1, p-value= 2.2e-16***) and growth regulators (X2= 20.91, df=4, p-value= 0.00032***). Leaf tissues were the most affected when 2,4-D (T29 was used in the culture medium (Figure 4). In the pith explants, treatments with BAP and 2, 4-D, promoted a higher contamination rate (T6 & T7) (Figure 5).
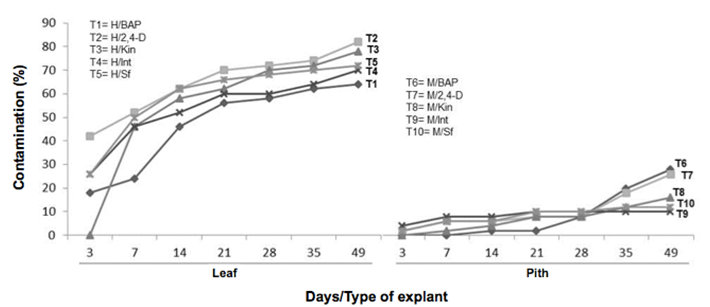
Figure 5 Dynamics of the contamination percentage in explants of Guarianthe skinneri. H: Leaf explant; M: Pith explant; Int: interaction of growth regulators; Sf: without growth regulators.
In general, the use of growth regulators increased the undifferentiated cell masses formation. The effect of cytokinins and auxins is the induction of cell division and differentiation (Huan et al., 2004). In particular, The BAP application generated better response in callus production (Figure 2). This type of response was observed by Thomas & Michael (2007), who obtained a better longitudinal development of Rhynchostylis retusa (L.) protocorms from the BAP combination (6 μM) and NAA (0.2 μM). Likewise, Chung et al. (2005), indicated when they used cytokinins, they obtained greater frequency of embryogenesis with leaf explants in Dendrobium 'Chiengmai Pink'. In this research, the highest callus production was achieved without hormone interactions, specifically using BAP. In general, it is not possible to ascertain whether separate employment or interaction with growth regulators is better; the answer also depends on other factors such as species, variety, type of explant, age of explant, cultivation condition, among others. Therefore, it is important to perform a suitable hormones combination to get the results of interest. Lee-Espinoza et al. (2010), reported the use of NAA auxin in combination with some cytokinin (BAP or Kin), decreased callus formation and multiplication rate in Laelia anceps ssp. dawsonii J. Anderson orchid, similar to the obtained in this research, due to leaf and pith treatments with the use of phytoregulators combination which, not generate significant results for the callus variable. The response rate generated by the use of BAP is due to the induction of cell division and increasing of meristematic cells. It is notorious that cytokinins are involved in the function of certain enzymes responsible for tubulin production, which is related to the mitosis and meiosis and play an important role in plant metabolism (Haq et al., 2013).
The type of explant and even its position within its organ influences the suitability in the callus production and/or somatic embryos. For example, Chen & Chang (2004), obtained different in vitro embryogenesis percentages when evaluating four leaf regions in Oncidium "Gower Ramsey". In our case, we could show the type of explant is a relevant factor to induce callogenesis in G. skinneri.
The highest percentage of callus formation was obtained when the type of pith explant was performed (Table 2). This is attributed to the composition of this type of explant, because of the large amount of reserve substance which have allowed the type of tissue. Ely et al. (2007), reported the orchid pseudobulbs are as follows: water, mineral and carbohydrate (starch) storage organs, as an adaptive response to the water, nutritional and solar radiation intensities which receive because they are epiphytes (Yen et al., 2008). These nutritional conditions of the pith tissue, would have favored the callus formation under hormones induction. At the same time, the starch presence in pith pseudobulbs, favored the oxidation with air and culture medium contact followed by the phenolization of the in vitro explants (Figure 3), since being a carbohydrate, it is sensitive to pH and temperature (Ely et al., 2007). In this research, a higher oxidation occurred when BAP was used. By contrast, Azofeifa (2009), and Chung et al. (2005), mentioned the auxins are the main phytoregulators related to the oxidation, due they stimulate the ethylene synthesis, which causes the tissue death. Likewise, there are reports in which it is mentioned this variable will depend on other factors such as type of explant, plant hormonal balance and the combination of these (Azofeifa, 2009; Van Staden et al., 2006).
In the present work, it was observed the explant type was statistically significant in the results of the variable contamination, registering a higher percentage of this variable in leaf fragments than in pith explants (Figure 5).
In spite of not finding reports about pseudobulb pith in orchids, the obtained results in this research indicated these explants might be an alternative for in vitro callus induction. Parada-Ponce & Villegas-Monter (2009), mentioned that, in addition to the type of explant, another factor, which influences the results of the contamination variable, is the explant size, they reported the smaller explant size decreases the contamination percentage. However, in our experimental conditions, this relationship was not observed. Therefore, it was considered more feasible the mother plants state of contamination (donor explant) was one of the causes of the obtained results. The above was attributed to the fact the orchids used as the mother plant, were obtained from several private households. They would be exposed to pollutants, which have allowed the plant production in a controlled environment. Among the characteristics considered for the mother plants collection, the age of the plants was not included. However, Kamali et al. (2001), indicated in vitro explants contamination varies according to the ages of mother plants and seasons of the year. Although different salt bases were not evaluated in the culture media in this study, there are reports in which, this factor influences the contamination percentages (Parada-Ponce & Villegas-Monter, 2009). This is attributed to the fact some media are more related to the development of microorganisms in nutritional terms.
Conclusion
Although pith explants had an oxidation of 100%, it was the type of explant, which generated low percentages of contamination and presented the greater callus formation. Therefore, the use of pith pseudobulb with BAP is an alternative of the callogenesis induction in Guarianthe skinneri, which could be used for in vitro development in terms of propagation protocols with tuning the protocol and limiting the observed oxidation.





![Corpoica Guayuriba 9: new improved soybean ( Glycine max [L] Merril) variety with specific adaptation to the Colombian foothills](/img/en/next.gif)