Introduction
The main citrus producing region in Northeastern Brazil comprises the states of Bahia and Sergipe, respectively the second and fourth national producers (Agrianual, 2013). The state of Sergipe produces mostly sweet oranges [Citrus sinensis (L.) Osbeck] (56000 ha), followed by 'Tahiti' acid lime [C. latifolia (Yu. Tanaka) Tanaka] (857 ha) and hybrids of mandarins (420 ha) (Agrianual, 2013). In Sergipe state, the majority of producers are smallholders which usually carry out low-input management practices. Citrus orchards are spread over 11000 farms located predominantly in the South of Sergipe state, within the range of the Coastal Tablelands, in properties with an average of less than 10 ha. The Coastal Tablelands lies along the coastline and is characterized by cohesive soils with low water availability during part of the year, rendering plants nutritionally unbalanced, underdeveloped, with low vegetative growth and yield (average 14 tons ha-1) and longevity (10-12 years of effective production) (Martins et al. 2014). In this region, the vast majority of farmers cultivate the sweet orange 'Pêra' [C. sinensis (L.) Osbeck] grafted on either Rangpur lime (Citrus limonia Osbeck) or Santa Cruz rangpur lime (C. jambhiri Lush.) (Prudente & Silva, 2009; Martins et al., 2014). This low genetic diversity of scion cultivars may lead to vulnerability to pests and diseases besides concentrating management practices and harvest within a given period which contributes to increasing costs. It is well known that pests reduce both yield and citrus orchard longevity (Gallo et al., 2002; Mendonça & Silva, 2009). Therefore, before introducing new scion cultivars of sweet oranges, mandarins and acid limes to this region it is important to evaluate its susceptibility to pests.
Pest populations are influenced by several abiotic environmental variables, i.e. temperature, relative humidity, wind, light, radiation, etc., as well as biotic ones, i.e. natural enemies and host plants (Gallo et al., 2002; Mendonça & Silva, 2009; Teodoro et al., 2009). Resistant cultivars are less damaged by pests in comparison with other cultivars in equal conditions owed to their genotype constitution (Vendramim & Guzzo, 2009, 2011). Due to the fact that they contribute for keeping pests below economic levels, besides being nontoxic to the environment and people and acting continuously against pests (Gallo et al., 2002; Vendramim & Guzzo, 2009; 2011), resistant plants are considered an important strategy of integrated pest management (Gallo et al., 2002; Chacón et al., 2012). Regarding citrus, it is possible that scion cultivars of sweet oranges, mandarins and acid limes respond differently to pests due to their genetic characteristics. Citrus orchards are attacked by a variety of pests including the citrus rust mite Phyllocoptruta oleivora (Ashmead, 1879) (Acari: Eriophyidae), the Texas citrus mite Eutetranychus banksi (McGregor, 1814) (Acari: Tetranychidae) and the tetranychid Tetranychus mexicanus (McGregor, 1950) (Acari: Tetranychidae). The citrus rust mite is a major pest in the main citrus producing regions of Brazil and its attack may lead to yield losses as well as aesthetic changes in fruit appearance and reduction in total soluble solids (Gallo et al., 2002; Moraes & Flechtmann, 2008; Mendonça & Silva, 2009). This mite feeds on leaves and on developing fruits, which surface turns silvery in lemons or rust brown in oranges as a result of attack (Moraes & Flechtmann, 2008; Mendonça & Silva, 2009). The Texas citrus mite E. banksi and T. mexicanus are considered pests of several crops including citrus (Quiros-Gonzales, 2000; Moraes & Flechtmann, 2008; Vacante, 2010). Colonies of Texas citrus mite are usually found on upper surfaces of leaves while colonies of T. mexicanus prefer to attack the underside of leaves. Leaves turn gray when infested by Texas citrus mite and yellow when attacked by T. mexicanus and may drop prematurely under heavy attack and stressful conditions such as drought. In contrast to P. oleivora, E. banksi and T. mexicanus rarely cause noticeable feeding damage on fruits (Quiros-Gonzales, 2000; Gallo et al., 2002; Moraes & Flechtmann, 2008; Vacante, 2010).
This research aimed to determine the buildup population of the citrus rust mite P. oleivora, the Texas mite E. banksi, and T. mexicanus over time in cultivars of sweet oranges, mandarins and acid limes grafted on Rangpur lime under field conditions of northeastern Brazil.
Material and methods
The experiment was conducted from June 2011 to February 2013 in a 3 year old citrus orchard installed in an Ultisol soil and located at the experimental area of Embrapa Coastal Tablelands in the municipality of Umbaúba, Sergipe state, Brazil (11°22'37'' S, 37°40'20'' W, 109 m. a. s. l.). The experiment consisted of twenty citrus scion cultivars (sweet oranges, mandarins and acid limes) grafted on Rangpur lime which is a widespread rootstock cultivar grown throughout the region. The scion cultivars were the sweet oranges 'Kona', 'Rubi', 'Natal CNPMF-112', 'Valência Montemorellos', 'Lima', 'Lima Succory Acidless', 'Lima Verde' and 'Pêra CNPMF-D6'; the hybrids of mandarins 'Piemonte' [Clementina mandarin (C. clementina hort. ex Tanaka) x 'Murcott'] and 'Murcott' (hibrid of unknown origin, possibly resulting from the crossing among tangerine and sweet orange; the mandarins 'Nova' and 'Page' [C. clementina x (C. paradisi Macfad. x C. tangerina hort. ex Tanaka)]; and the 'Tahiti' acid lime [C. latifolia (Yu. Tanaka) Tanaka] clones 'CNPMF-01', 'CNPMF-02', '5059', 'IAC 5', 'IAC 5-1', 'CNPMF-2001', 'Persian Lime 58' and 'Bearss Lime'. The sweet orange 'Pêra CNPMF-D6' was included for comparison as it is the main scion cultivar grown in the region. The experiment consisted of a completely randomized block design with three replications and three plants per plot and mites were evaluated only on the central plant to avoid influences of neighboring cultivars.
The experiment was managed as a commercial orchard with management practices such as fertilizing, pruning, weed and pest control since it is part of a greater project aiming at selecting scion cultivars for the Brazilian northeast. Pesticide spraying was carried out over the period of pest evaluation using mancozebe (April, 2011); imidacloprid (June, September and December, 2011; August and December, 2012 and February, 2013), deltamethrin (October, 2011); vegetal oil (October and December, 2012) and mineral oil (February, 2013). Pesticides were equally applied to plants of the experiment.
Adults of citrus rust mite P. oleivora, Texas citrus mite E. banksi, tetranychid T. mexicanus and those of the predatory mite Iphiseiodes Iphiseiodes zuluagai Denmark & Muma, 1972 (Acari: Phytoseiidae) were monthly counted from April 2011 to February 2013 in a 1cm2-area. The predatory mite I. zuluagai was also counted because it is an important naturally-occurring predator of pest mites commonly found in citrus plants (Moraes & Flechtmann, 2008). This is a generalist predatory mite feeding on pest mites as well as on alternative food sources such as pollen, nectar and honeydew and it occurs throughout the year in citrus orchards of Sergipe state. For P. oleivora, two randomly-selected fruits were evaluated per plant totaling six fruits per cultivar in each evaluation. Care was taken to select only fruits located in the external part of the plant since this region is the most attacked by P. oleivora (Mendonça & Silva, 2009). The mites E. banksi, T. mexicanus and I. zuluagai were evaluated in four randomly-collected leaves per plant totaling 12 leaves per treatment in each evaluation.
Repeated Measures ANOVAs followed by post hoc Fisher LSD tests were conducted to determine the effect of scion cultivars of sweet oranges, mandarins and acid limes on population densities of the citrus rust mite P. oleivora, the Texas citrus mite E. banksi and T. mexicanus over the evaluation period. ANOVAs followed by post hoc Fisher LSD tests were carried out to compare the populations of either E. banksi or T. mexicanus among scion cultivars within each month. Whenever necessary, data were log x+1 transformed prior to analyses.
Hierarchical partitioning analyses were performed to evaluate the relative contribution of the variables temperature, relative humidity, rainfall, radiation, and I. zuluagai on the abundance of P. oleivora, E. banksi, and T. mexicanus using the software R with "hier.part" and "gtools" packages (Mc Nally & Walsh, 2004). This analysis estimates the percentage of explained variance of each factor in joint and independent contributions with all other factor, considering all possible models in a multivariate regression (Mc Nally & Walsh, 2004; Teodoro et al., 2008). Pearson correlations among mean temperature, relative humidity, rainfall, radiation and population densities of P. oleivora, E. banksi, T. mexicanus and the predatory mite I. zuluagai were conducted. Linear regression analyses were conducted between significant correlations. Mean temperature (°C), mean relative air humidity (%), and radiation (MJ.m-2) data were obtained from the database of the National Institute of Meteorology (INMET) and rainfall (mm.month-1) data were collected in the database of the National Institute for Space Research (INPE).
Results and discussion
The abundance of the citrus rust mite P. oleivora was not influenced by scion cultivars (df = 19, 40; F = 1324; P = 0.222) indicating that all twenty cultivars are equally susceptible to this pest, however the number of P. oleivora varied over time (df = 12, 480; F = 20.49; P < 0.0001). Population levels of the citrus rust mite peaked in April, June and November 2011 and in February 2012 (Figure 1). In this region, the citrus rust mite normally occurs from September to January, period of low rainfall and high temperatures (Mendonça & Silva, 2009). There was no interaction between time and scion cultivars in the abundance of P. oleivora (Figure 1; df = 228, 480; F = 1172, P = 0.076). This contrasts with a study by Moraes et al., (1995), which found a higher incidence of the citrus rust mite on leaves of the sweet orange 'Seleta Franck' in comparison with sweet oranges 'Hamlin' and 'Valencia' in the southern Brazilian state of Santa Catarina.
The abundance of the Texas citrus mite E. banksi was neither influenced by scion cultivars (df = 19, 40; F = 1.219; P = 0.290) nor by time (df = 3, 120; F = 1.047; P = 0.374). However, there was an interaction among time and scion cultivars in the abundance of E. banksi (Figure 2; df = 57, 120; F = 1497, P = 0.033) in that higher populations of this pest were observed in February 2012, on sweet orange 'Valência Montemorellos' in comparison to the remaining cultivars (including the prevailing scion cultivar in this region, i.e. 'Pêra CNPMF-D6').
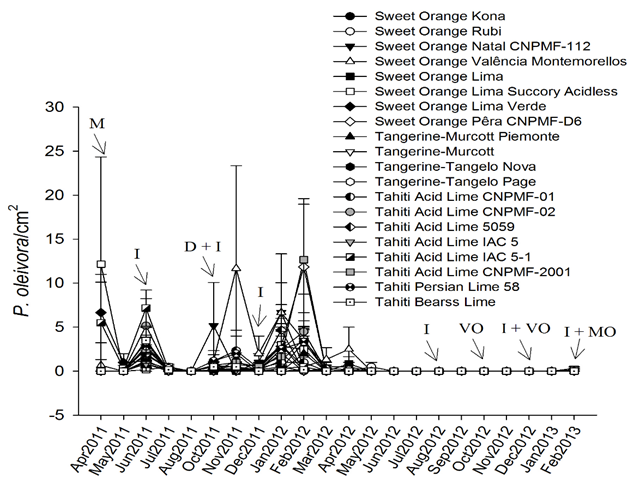
Means ± SE are presented (untransformed data). Arrows indicate spraying with imidacloprid (I), mancozebe (M), deltamethrin (D), mineral oil (MO), and vegetal oil (VO).
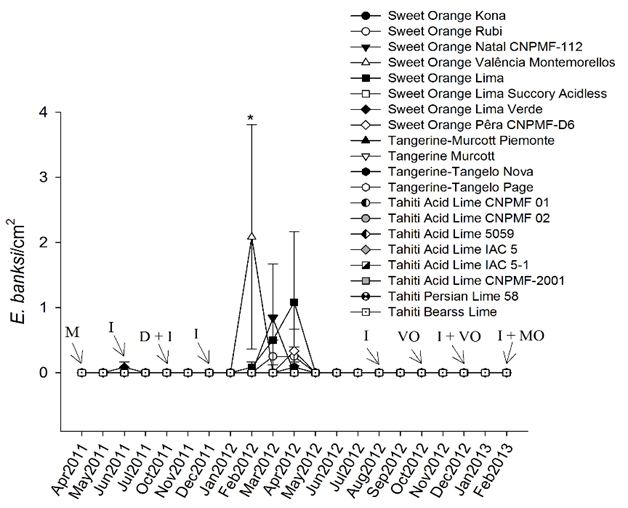
Figure 2 Seasonal changes in the abundance of the Texas citrus mite Eutetranychus banksi on leaves of twenty scion cultivars grafted on Rangpur lime.
Means ± SE are presented (untransformed data). Asterisk represents significant difference in the abundance of E. banksi among scion cultivars in February 2012. Arrows indicate spraying with imidacloprid (I), mancozebe (M), deltamethrin (D), mineral oil (MO), and vegetal oil (VO).
Populations of the pest mite T. mexicanus were not influenced by scion cultivars (df = 19, 40; F = 1097, P = 0.388). However, time influenced the abundance of T. mexicanus with a peak in January 2013 (Figure 3; df = 11, 440; F = 16.50; P < 0.0001). Also, there was an interaction between time and scion cultivars in the abundance of T. mexicanus (df = 209, 440; F = 1227; P = 0.039) in that higher populations were found in sweet orange 'Lima' in January 2013 in comparison to 'Tahiti' acid limes IAC 5 and IAC 5-1 (Figure 3). 'Pêra CNPMF-D6' and the remaining cultivars had intermediate population levels of T. mexicanus (Figure 3). Scion cultivars grafted on Rangpur lime affected populations of the pest mites E. banksi and T. mexicanus in some months, contrasting to the no influence of these cultivars on the abundance of the citrus rust mite P. oleivora, and suggesting possible resistance mechanisms to those tetranychids in some cultivars.
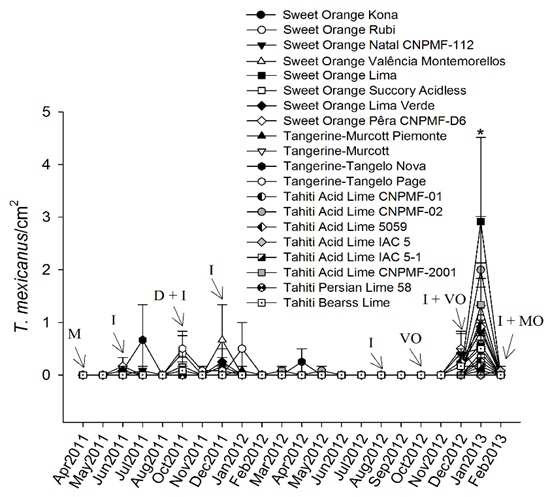
Figure 3 Seasonal changes in the abundance of the mite Tetranychus mexicanus on leaves of twenty scion cultivars grafted on Rangpur lime.
Means ± SE are shown (untransformed data). Asterisk indicates significant difference in the abundance of T. mexicanus among scion cultivars in January 2013. Arrows represent spraying with imidacloprid (I), mancozebe (M), deltamethrin (D), mineral oil (MO), and vegetal oil (VO).
In addition, the experiment was carried out as a commercial orchard, reflecting the region management practices, pesticide spraying may have negatively influenced evaluations of the populations of P. oleivora, E. banksi and T. mexicanus (Figures 1, 2, 3). For example, there was a sharp reduction in the number of P. oleivora in May, July and August 2011, probably due to pesticide spraying in previous months (Figure 1). Also, high populations of E. banksi were only found in periods without pesticide spraying (February and April 2012) (Figure 2) suggesting that this mite is highly susceptible to these chemicals. This may be due to the intrinsic susceptibility of this species to pesticides as well by the fact that E. banksi colonies are found on the upper side of leaves which is more exposed to pesticides. Colonies of T. mexicanus, on the other hand, are found mainly on the underside of leaves. Pesticide spraying also seemed to drastically reduce populations of T. mexicanus (Figure 3). In the case that the study had been conducted without the application of pesticides the results could have been more consistent, however all pesticides were equally sprayed in all treatments and therefore not invalidating our results.
The relative contributions of the abiotic and biotic variables differed markedly among the pest mite species. Most of the variance for the abundance of P. oleivora was explained by the abundance of the predatory mite I. zuluagai (63.7%), followed by temperature (17.8%), relative humidity (11.0%), rainfall (4.7%), and radiation (2.2%) (Figure 4). For E. banksi, most of the variance was explained by radiation (39.5%), relative humidity (28.8%), and rainfall (19.1%) while the remaining variables explained lower portions of variation (Figure 4). For T. mexicanus, temperature alone explained over 75% of the variation while relative humidity, rainfall, radiation and I. zuluagai explained less than 25% of the variation. However, except for the abundance of T. mexicanus and temperature (Figure 5; rp = 0.468, P = 0.032) all remaining correlations were not significant (Pearson correlations: rp < 0.407, P > 0.05). This correlation corroborates hierarchical portioning results and it indicates that the population of T. mexicanus increases in periods of high temperature (Figure 5) which is line with other studies showing that tetranychid mites are generally positively influenced by temperature (Praslicka & Huszar, 2004; Teodoro et al., 2009). Although hierarchical partitioning analyses indicated that relative humidity and rainfall explained significant portions of the variance for E. banksi, these abiotic variables were not correlated with populations of any pest mite in this study. The abundances of the pest mites were also not correlated with radiation and the population of the generalist predatory mite I. zuluagai, although this predator is abundant and naturally occurs in citrus orchards and it was common during the period of our evaluations. This contrasts with hierarchical partitioning results showing that I. zuluagai explained over 60% of the variance for P. oleivora.
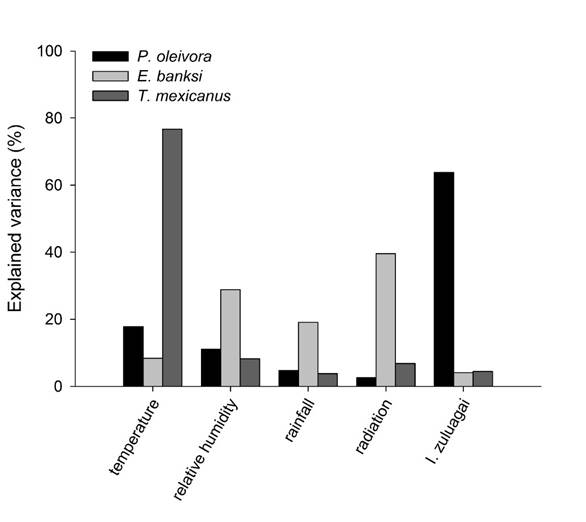
Figure 4 Percentage of explained variance of biotic and abiotic variables for the abundance of the pest mites Phyllocoptruta oleivora, Eutetranychus banksi, and Tetranychus mexicanus.
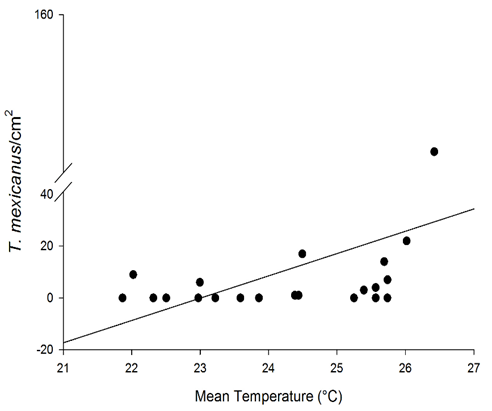
Figure 5 Relationship among the population of the mite Tetranychus mexicanus and mean temperature (y = -10.003 + 0.459x; R2 = 0.21, df = 1, 19; F = 5.337, P = 0.032). Untransformed data are shown.
Besides the abiotic environmental variables like temperature, relative humidity, rainfall, radiation, and natural enemies (Praslicka & Huszar, 2004; Vis et al., 2006; Teodoro et al., 2009), pests can also be affected by resistance of different genotypes. To some extent, our results suggest the existence of putative antibiosis and/or antixenosis (Kogan & Ortman, 1978; Vendramim & Nishikawa, 2001) as possible mechanisms involved in the resistance of some scion cultivars to the tetranychid mites E. banksi and T. mexicanus, but additional studies are necessary to confirm it. In a parallel research, we showed that abundances of the mites P. oleivora and T. mexicanus on the sweet oranges 'Pêra CNPMF D-6' and 'Valencia Tuxpan' were affected in some periods of the year by rootstocks, indicating also the influence of rootstock genotypes on these pests (Martins, Teodoro & Carvalho, 2014).
Conclusion
The abundance of the Texas citrus mite E. banksi and the tetranychid T. mexicanus were influenced by scion cultivars only in some periods, indicating putative resistance mechanisms in some genotypes to these pests. In contrast, the abundance of the citrus rust mite P. oleivora was not influenced by scion cultivars over the evaluation period. Abiotic and biotic variables were important mechanisms underlying such responses.














