Introduction
Sugarcane planting requires specifically designed farm machinery for its establishment, management, and harvest. It is an intensive labor and requires around 12 to 18 t ha-1 of vigorous seed and complete stalks (Viveros, Baena, Salazar, López & Victoria, 2015 ; Abd, El Mawla, Hemida & Mahmoud, 2014). In recent years, there has been a growing interest in mechanized sugarcane planting, a process that utilizes sugarcane chips from previous mechanized harvests. Even though the process has been around for a decade developed with the introduction of precision planters, it has not been possible to achieve either reduction of sugarcane seed amount or a proper harvesters management, which damage the seed and, consequently, germination ability (Ripoli & Ripoli 2010; Riascos, Espitia & López, 2015). Planting failures result in problems such as weeds, untapped consumables, and low sugarcane yield. As an option to tackle this, a greater amount of seed is used to provide enough healthy buds and to ensure a planting as uniform as possible (Siqueira, Pierre, El Tahchy, Glassop, Singh, Bonnett & Rae, 2015).
Recently, mechanized planting system using seedlings has been evaluated at the Agricultural Genetic Engineering Institute in Coimbatore. Results showed a reduction in planting costs by 40% compared to conventional cost (Naik, Annamalai, Vijayan & Rajendra, 2013). Nevertheless, system is still laborious and slow, requires a great deal of plant material preparation (e.g., seedlings). Furthermore, there is a greater speed in sugarcane sowing operation, as well as a certain uniformity of seedling depth placement, which enables a similar crop development.
Such attempts have not solved the need for precision mechanized sowing, which reduces the quantity labor as well as the volume and weight of the required seed, making sowing faster. A possibility to achieve this purpose is using encapsulated sugarcane buds (artificial seed). Utilization of artificial seeds in other crops has been demonstrated to be a viable strategy in their propagation for commercial purposes, favoring the management of natural resources in sustainable form (Nieves, Zambrano, Tapia, Cid, Pina & Castillo, 2003).
The term artificial seed, generally describes a somatic embryo or shoot, encapsulated with a synthetic covering for protecting it from the environment and mechanical damage; additionally, the covering contribute with nutrients and be sufficiently soft to allow germination and exchange of gases as well as the respiration of the propagule (Morales & Cano 2012).
The aim of this study was to develop artificial sugarcane seed CP-54 using polymeric materials for encapsulation, protect the shoot from damages during handling, and to evaluate seed germination after 20 and 45 days after planting (dap), in addition, analyze plant growth and development of sugarcane seedlings, physical condition and encapsulation of artificial sugarcane seed CP-54 after planting.
Materials and methods
Study area
This work was carried out at the experimental field of Colegio de Postgraduados, Campus Tabasco, Mexico. Plant material was taken from sugarcane plantations of eight months old, located at Poblado C-34 (Presidente Benito Juárez García) in Tabasco (coordinates: 17° 58' 16" N, -93° 27' 30" W). In order to fulfill the aim of this study, the study was divided into two stages.
Obtaining of sugarcane buds
Sugarcane stalks were cut with a sharp machete, subsequently, a hand saw was used to cut 35 mm portions of shooted stalk, with 25 mm of reserve from lower scar and 15 mm from upper side, conversely, roots zone and shoot were not damaged.
Stalks disinfection
Shoot chips were disinfected by immersion into fungicide-insecticide solution of Malathion 50 EC (trident agrochemical) at 0.1% and Carbendazim (Prozycar® 500) at 0.1%. Buds were submerged for ten minutes and subsequently, have allowed drying at air for ten minutes.
Encapsulation of sugarcane buds
Four polymers were evaluated where each encapsulation was approximately 5 mm in thickness. Starch: 100 g of cornstarch (Maizena®) were used along with 1 L of water. In a beaker (KIMAX®,), 750 mL of water was heated on a hotplate (Thermo Scientific). Cornstarch was dissolved in 250 mL ofwater in a separate container and 750 mL of hot water was added; mixture was agitated until complete homogenization, then 400 g of ground-dried straw was weighed using a scale (TJ611 model, OHAUS®) and poured within a plastic container. Grounded straw was placed into a plastic tray and starch mixture was then added to create a paste. Buds were manually covered with a capsule formed, and were left to dry for 72 hours under shade conditions.
Polymers A factorial design of3 x 4 was established as follows: three cultivars (MEX 69 290, MEX 68-P-23, and CP 72-2086) and four polymers (starch, gelatin, sodium polyacrylate, and sodium alginate). Combination of these factors generated 12 treatments, which were evaluated by a completely randomized design, with 50 replicates each one.
Gelatin. 250 g of gelatin (Duche®) were mixed with 1 L of water in a beaker. Mixture was heated using a hotplate to eliminate clumps and to homogenize. When mixture became homogenized, was removed from heat and had achieved a decreasing in temperature to 32°C in order to prevent shoot damages. Gelatin mixture was poured onto a plastic tray and 100 g of ground-dried straw was added to form a paste. Buds were submerged into gelatin and straw mixture, which were placed on a plastic tray and thereafter covered with additional ground-dried straw. Encapsulations were left to dry on a plastic tray for 72 hours under shade conditions.
Sodium polyacrylate. 400 mL of common white glue (Bully®) were mixed with 100 mL of water in a beaker. Buds were manually covered with this mixture and thereafter placed on a plastic tray to be covered with sodium polyacrylate (Sigma-Aldrich®). Encapsulations were left to dry for 72 hours under shade conditions.
Sodium alginate and calcium chloride. 20 g of sodium alginate (MEYER®) mixed with 1 L of water in a beaker and constantly agitated to avoid clumps formation. In the same way, 112 g of calcium chloride (J.T. Baker®) mixed with 1 L of water in a beaker. Beaker containing a sodium alginate mixture, 300 g of dry ground dried-straw were added to form a paste. Buds manually covered with this paste until a capsule formed. Capsules submerged into calcium chloride solution for five minutes in order to solidify polymer and placed on a plastic tray for 72 hours under shade conditions.
Study variables
Physical state of artificial sugarcane seed CP-54. Visual analysis of physical state of the capsules after 72 hours of repose was made as follows 1= encapsulation without deformities or damage, 0= encapsulation with covering, germination, or fungi growth detachment.
Evaluation of artificial sugarcane seed CP-54 germination. In order to evaluate treatments, a randomized design with a 3x4x4 factorial arrangement was used as follows: three cultivars (MEX 69-290, MEX 68-P-23 and CP 72-2086); four polymers (starch, gelatin, sodium polyacrylate and sodium alginate with calcium chloride); and four shoot positions in the substrate (12, 3, 6 and 9 clockwise). It generated 48 treatments, which were distributed on trays in an array completely randomized with 10 replicates. Germination counting of artificial sugarcane seed CP-54 after 10, 15, 20, 30 and 45 days of planting were registered. Only results concerning days 20 and 45, are presented in this study.
Substrate preparation. 200 L of river sand, which was washed five times with running water to remove salts and impurities, was used a substrate.
Artificial seed planting. A shallow concrete made box was divided into 10 parts of120 cm wide and 80 cm long was filled up to one quarter with substrate. Four lines were marked on substrate, and thereafter, 12 encapsulations were placed in each line to complete a treatment replicate. Subsequently, encapsulations were covered with 5 cm of sand. Finally, light irrigation was applied to keep moisture level in substrate with approximately 4 L of running water, irrigation need was daily determined.
Seedling height and root length. After 45 days of planting, sugarcane seedlings were extracted and seeds that did not germinate, in order to evaluate the shoot condition, determine the seedling height, and root length. Boxes were saturated with running water to soften substrate and were then carefully extracted to avoid breaking roots. Longest plant root and stalks height were measured from plant base to top leaf.
Shoot physical condition. At time of seedling extraction, a visual evaluation of shoot physical condition was made. For such purpose, a scale was used as follows: 1= seeds germinated and/ or shoot still alive; 0= buds damaged or dead.
Encapsulation physical state. A visual evaluation was made with the following scale: 1; encapsulation remained on seed, 0; encapsulation fully disintegrated.
Statistical analysis
Each trial were analyzed using the GLM procedure from Statistical Analysis Systems, SAS software, version 9.3. To identify significant difference among treatments and statistical significance for all comparisons was made at p<0.05. Tukey's multiple range test was used to compare the mean values of treatments.
Results
Germination of artificial sugarcane seed CP-54 after 20 days of planting
ANOVA showed significant effects for polymers and interaction between P*Po (Table 1). Coefficient of variation was 83.9%, which is considered high. Such result can be attributed to polymers use for shoot coating. According to Tukey test, germination was statistically equal in three cultivars, with an average germination of 50% (Table 1).
Table 1 Average germination of artificial sugarcane seed CP-54 at 20 and 45 dap
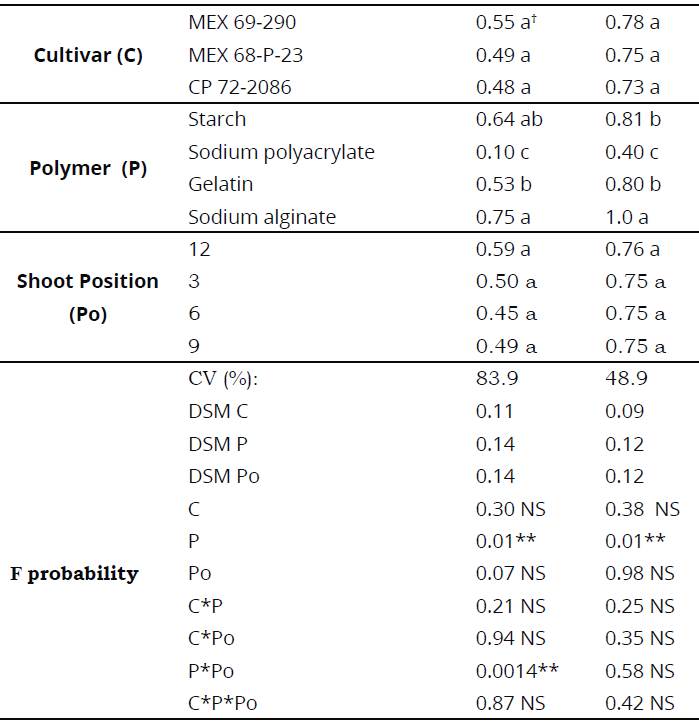
*Reported mean values ± standard deviation. Values with different letters within the same column, are significantly different based on Tukey test (P<0.05).
A statistical significance of the interaction described above indicates that sodium polyacrylate presented the lowest germination after 20 days, which ranged from 0-20%, independent of shoot position or used cultivar.
Germination of artificial sugarcane seed CP-54 after 45 days of planting
ANOVA indicates significant effects only for polymers, but not for cultivars, shoot positions or any interactions (Table 1). Coefficient of variation was 48.9%, which is considered high in seed germination after 45 days. Therefore, can be attributed to polymers effect that delayed germination. According to Tukey test, germination was statistically equal in the three cultivars (Table 1).
Artificial sugarcane seed CP-54 elaborated with sodium alginate presented a germination percentage of 100%, outweighing artificial seeds elaborated with starch and gelatin.
Figure 1, shows physical condition of sugarcane plants germinated from different positions. Position 6 would usually be the most adverse position to sprout, but in this case, sugarcane seedlings obtained with greater weight, which implies that any artificial sugarcane seed placed in soil will germinate and shoot, will sprout.

Figure 1 Physical state of sugarcane seedlings 45 dap showing plant development for different shoot position. a) sugarcane seed tillering with a shoot; b) seedling with shoot located at position 3 located at position 12 when planted; c) seedling with shoot located at position 6; d) seedling with shoot located at position 9.
Root length at 45 dap
ANOVA analysis for root length indicates significant effects for polymers, and interactions C*P and P*Po (Table 2). Coefficient of variation was 72.06%, which is considered high in root length measurement. It can be attributed to polymers, nutrition, and cultivar. Artificial sugarcane seed CP-54 elaborated with sodium alginate, starch, and gelatin polymers, presented a greater root length compared to seed treated with sodium polyacrylate, since these polymers have allowed seed germination and root growth. A significance of C*P interaction indicates that root length was greater in artificial sugarcane seed elaborated with starch and sodium alginate with calcium chloride, in the three cultivars (Table 2).
Table 2 Root length, seedlings height, physical condition of the shoot and encapsulation on the artificial sugarcane seed CP-54 at 45 dap
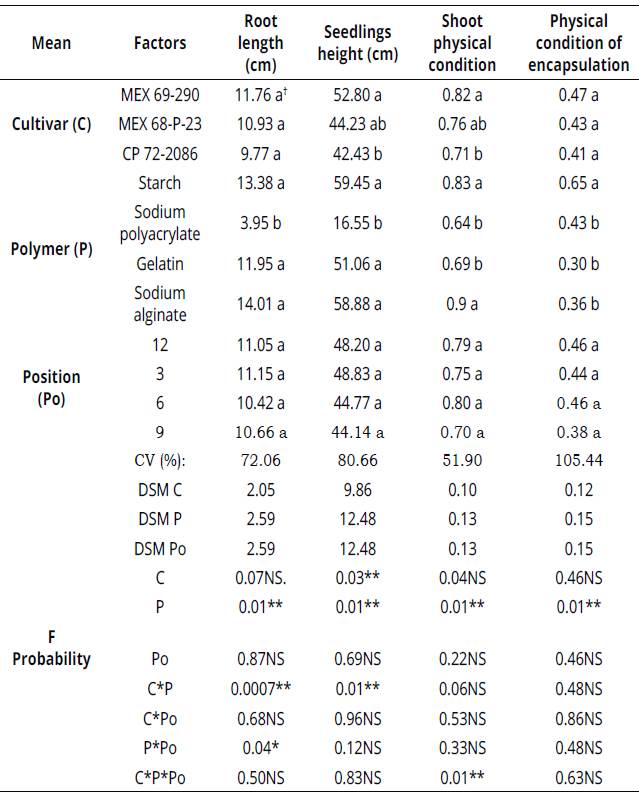
*Reported mean values ± standard deviation. Values with different letters within the same column, are significantly different based on Tukey test (P<0.05).
The significance of P*Po interaction shows that artificial sugarcane seed CP-54 elaborated with starch and sodium alginate plus calcium chloride, presented a greater root length compared to gelatin and sodium polyacrylate (Table 2).
Plant height at 45 dap
ANOVA analysis for seedling height showed significant effects for cultivar, polymers, and C*P interaction (Table 2). Coefficient of variation was 80.6%, which is considered high in plant height measurement. It can be attributed to the fact that some polymers delayed seed germination and plant development.
According to Tukey test, cultivar CP 72-2086 presented the lowest plant height (Table 2). Some of the polymers used to elaborate the artificial seed delayed its development. Conversely, cultivars MEX 69-290 and MEX 68-P-23 presented the greatest plant height. Artificial sugarcane seed CP-54 elaborated with the polymers starch, gelatin, and sodium alginate presented a greater plant height (Table 2). The significance of C*P interaction indicates that plant height was greater in the artificial seed elaborated with starch and sodium alginate and calcium chloride, in the three cultivars (Table 2).
Physical condition of the artificial sugarcane seed CP-54 at 45 dap
ANOVA results for shoot physical condition in the artificial sugarcane seed CP-54 show highly significant differences for polymer, cultivar effects and the following interactions: C*P and C*P*Po (Table 2). Coefficient of variation was 51.9%, which is considered high. However, is explained by a poor performance of polyacrylate to encapsulate artificial sugarcane seed.
Tukey test indicates that buds in artificial sugarcane seed from MEX 69-290 and MEX 68-P-23 cultivars remained significantly better compared to buds from cultivar CP 72-2086. The significance of C*P*Po interaction indicates that physical condition of buds were better in artificial sugarcane seed elaborated with starch and so dium alginate polymers with calcium chloride in the three cultivars (Table 2), regardless of shoot position.
Physical condition of encapsulation at 45 dap
ANOVA results for the encapsulation of artificial sugarcane seed indicate significant differences among polymers, but not for cultivar and its interactions (Table 2). Coefficient of variation was 105.4%, which is considered high. It can be attributed to encapsulation and to a poor performance of some polymers that turned to be fragile. A significant effect of polymer indicates that encapsulation with starch keeps the shape of artificial seed without interrupting seed germination and seedlings development (Figure 2).
Discussion
Polymers that presented a higher percentage of germination were sodium alginate and starch, with an average germination of 75% and 64% respectively. With 10%, sodium polyacrylate presented the lowest germination percentage. With respect to shoot position in the substrate, did not appear to have influence on germination, since all positions presented 50% germination and agrees with what was previously reported by Álvarez, Salgado, Córdova, Castelán, Ortiz, García & Castañeda (2016).
With such germination, any of these seeds may suffice for precision mechanized planting of sugarcane. Artificial sugarcane seed elaborated with sodium polyacrylate presented the lowest germination percentage of 40%, due to the water absorption properties that delayed germination. With respect to shoot position in the substrate, did not seem to influence seed germination, since it shows results of 75% of seed germination in any shoot position (Table 1).
These results are comparable in variability to the report by Mehpara, Mujib & Siddiqui (2012), in a study carried out with synthetic seed of Catharanthus roseus (L.) G. Don, where was evaluated its plant development and conversion to plantlet.
Currently, there has not been any published report related to germination of artificial sugar cane seeds, aside from an Indian report on germi nation of 80% when sugarcane was planted from shoot chips stored for 10 days at a low tempera ture (Radha, Solomon, Shrivastava & Chandra, 2010). Given these concerns, this study could be considered pioneer in this context.
In fact, in previous work Lal, Tiwari & Gupta (2015); Nieves, Zambrano, Tapia, Cid, Pina & Castillo (2003), they argue that there has been considerable interest in applying these tech niques in breeding, propagation, disease elimina tion, rejuvenation of older varieties, development of clones suitable for abiotic and biotic stresses, conservation of genetic resources etc., is now drawing special attention for multiplication of new varieties on large scale in comparatively shorter period of time in several crops including sugarcane.
Encapsulation displays several advantag es: easy handling of samples, simplification of cryoprotective media, elimination of costly pro grammed freezers in most cases, and increased size of explants surviving liquid nitrogen storage (Gupta, 2014; Rafique, Yamamoto, Fukui, Tana-ka, Valle, Arizaga, Abbas, Matsumoto & Niino, 2016).
The encapsulation-dehydration technique comprises the following steps: pre-treatment; encapsulation; preculture; desiccation; freezing and storage; thawing and regrowth. On the other hand, in previous study carried out by González & Engelmann (2006), sugarcane apices were excised, left overnight on standard medium to recover from the dissection stress, and then en capsulated in 3% (w/v) calcium alginate beads, alginate-coated apices of sugarcane resumed growth more slowly than nonencapsulated controls.
In this study, was observed that artificial seeds elaborated with starch and sodium alginate, presented a significantly physical condi tion compared to encapsulation of gelatin and polyacrylate; former turned out to be a fragile encapsulation without consistency, and latter, delayed seed germination and plant development due to its water absorption properties; these provides more accurate and reliable estimates of encapsulation.
Conclusion
The polymers used to encapsulate the artificial sugarcane seed CP-54 enable seed germination, root zone growth and development of sugarcane plants. They also keep encapsulation in a good physical condition. Given these concerns, greatest germination of the artificial sugarcane seed CP-54 was obtained with starch and sodium alginate encapsulation in shoot positions 12, 3 and 9 regardless of the cultivar.















