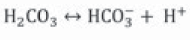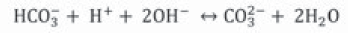Introduction
Biomineralization is a process carried out in nature that involves organisms that produce mineral precipitation in their cellular activity (Phillips et al., 2013b; Stocks-Fischer, Galinat & Bang, 1999b), as is the case with silicates in algae and especially diatoms, carbonates in invertebrates and phosphates in vertebrates (Dhami, Reddy & Mukherjee, 2014); this leads to the formation of more than 60 biological extracellular and intracellular mineral products (Sarikaya, 1999). There are two types of mineralization: biologically controlled mineralization (BCM) and biologically induced mineralization (BIM). In the first, minerals are normally deposited within organic matrices or vesicles in living cells, allowing organisms to exert a significant degree of control over the nucleation and growth of minerals (Weiner & Addadi, 1997). In the second, the microorganisms secrete one or more metabolic products that react with the ions or compounds in the environment, with the subsequent deposition of the mineral as a metabolic byproduct (Frankel, 2003). From a sustainable construction point of view BIM is the most important and studied type (Achal, Mukherjee, Kumari, & Zhang, 2015).
Precipitation of calcium carbonate induced microbiologically (MICP)
Calcium carbonate is one of the most common minerals on earth comprising in weight ca. 4 % of the crust of the earth, and its precipitation occurs naturally in marine and freshwater as well as in soils (Castanier, Le Metayer-Levrel & Perthuisot, 1999). This process occurs mainly due to an increase in its concentration or a decrease in solution solubility, causing evaporation, changes in temperature or pressure, and biomineralization. Precipitation of calcium carbonate induced microbiologically (MICP) refers to the formation of calcium carbonate from an oversaturated solution due to the presence of microorganisms and their biochemical activities (Bosak, 2011). During MICP, organisms secrete one or more metabolic products (CO3 -2) that react with ions (Ca2+) found in the environment with subsequent mineral precipitation (Zhu, Li, Zhan, Huang, Zhang & Achal, 2016b); such substances act as cementing materials and are commonly known as "biocement" (Rong, Qian & Li, 2012). Several works have demonstrated the existence of mechanisms that form calcium carbonate such as photosynthesis (McConnaughey et al., 1997), urea hydrolysis (De Muynck, Verbeken, De Belie, & Verstraete, 2010b; Dhami, Reddy & Mukherjee, 2013b; Galinat & Bang, 1999a; Stocks-Fischer,), anaerobic sulfides oxidation (Warthmann, Van Lith, Vasconcelos, McKenzie & Karpoff, 2000), and by extracellular polymeric substances (Arias & Fernández, 2008; McConnaughey et al., 1997). However, the most widespread method for calcium carbonate precipitation is urea hydrolysis (DeJong, Mortensen, Martinez & Nelson, 2010; Hammes et al., 2003b; Hammes, Verstraete & Verstraete, 2002).
Ureases of bacterial origin
Ureases (urea amidohydrolases, E.C. 3.5.1.5.) are a group of enzymes that hydrolyze urea producing carbon dioxide and ammonia, involving an increase in pH. One of the main characteristics of this family of enzymes is the presence of metallic centers in their active sites; these activate substrates and water for the reaction. Regarding urease, nickel ions (Ni (II)) stand out in its active site (Krajewska, 2009). An important function of these enzymes is to promote biomineralization in nature, allowing the precipitation of calcium carbonate in soils, geological sediments and natural waters (Mobley & Hausinger, 1989). Although there is a wide variety of microorganisms with urease activity, the Bacillus group is known for its high levels of urease (Achal et al., 2015), especially Sporosarcina pasteurii, formerly Bacillus pasteurii (21 mM hydrolyzed urea.min1) (Achal et al., 2015; Dupraz, Parmentier, Ménez & Guyot, 2009; Ferris, Phoenix, Fujita & Smith, 2004); this soil bacteria is non-pathogenic and grows at an optimum pH of 9.0 tolerating extreme conditions, and therefore, it has been used for MICP (Bang, Galinat & Ramakrishnan, 2001; Hammes et al., 2003b; Kumari, Pan, Lee, & Achal, 2014; Mitchell & Ferris, 2005). It has been shown that urease activity in bacteria is associated with soluble extracts in cells, which would indicate that the enzyme is in the cytoplasm of the microorganisms (Mobley & Hausinger, 1989). The ideal microbial source of ureases will be one that supports high concentrations of urea and calcium, as well as having a high urease activity that is constitutive or can be induced, i.e. the enzyme is produced independently of environmental conditions or the expression of urease is induced by the presence of urea (Mobley, Chippendale, Swihart & Welch, 1991). Bacteria that produce urease can be classified into two groups according to their response to ammonium: those whose urease activity is not affected by the presence of inhibitors such as ammonia (e.g. Sporosarcina pasteurii, proteus vulgaris, proteus mirabilis and Helicobacter pylori), and those that are affected (e.g. Pseudomona aeruginosa, Alcaligenes eutrophus, Bacillus megaterium and Klebsiella aerogenes) (Mulrooney, Zakharian, Schaller & Hausinger, 2001; Wiffin, 2004). In addition, MICP can provide high concentrations of calcium carbonate in a short period of time (Dhami, Reddy & Mukherjee, 2013a). Urease influences the mineral formation process by four factors: concentration of calcium ions, dissolved inorganic carbon ratio, pH and presence of nucleation sites (Rong et al., 2012), the latter of great importance for continuous and stable calcite crystals formation (Phillips et al., 2013b); however, in the case of Biomineralization, it is carried out by bacteria that on their cell surface, which are charged with negative groups, divalent cations are anchored (Ca2+ or Mg2+) at a neutral pH, making them ideal nucleation sites for calcite deposition (Ferris, Stehmeier, Kantzas & Mourits, 1996; Ramachandran, Ramakrishnan & Ban, 2001; Stocks-Fischer et al., 1999b,). However, calcium ions bond more frequently than magnesium ions because they have a stronger ionic selectivity (Sánchez-Román, Rivadeneyra, Vasconcelos & McKenzie (2007). Therefore, bound cations (metal ions) react with anions (carbonates) to form insoluble calcium carbonate (De Muynck et al., 2010b). Bacterial cells play a key role in the MICP because, in addition to being used as nucleation sites, they affect the type of mineral that is going to be formed (Douglas & Beveridge, 1998; Rodriguez-Navarro, Jroundi, Schiro, Ruiz-Agudo & Gonzalez-Muñoz, 2012).
Calcium carbonate precipitation mechanism
Urease catalyzes urea hydrolysis to produce ammonium and carbonate. In this reaction, one mole of urea is hydrolyzed and forms one mole of ammonium and one mole of carbamic acid (Equation 1), which is hydrolyzed spontaneously to another ammonium and carbonic acid molecule (Equation 2) (Hammes et al., 2003a; Li et al., 2000; Stocks-Fischer et al., 1999b), which are balanced in an aqueous medium and forms bicarbonate (Equation 3), two moles of ammonium and two moles of hydroxyl ions (Equation 4). The latter increases pH medium changing the equilibrium of bicarbonate with subsequent carbonate ions formation (Equation 5) (Fujita et al., 2008); this change precipitates metal ions. The generation of NH4 + increases the pH of the medium and the reaction continues spontaneously towards calcium carbonate formation (Ferris et al., 1996; Mitchell & Ferris, 2005) on the surface of the bacterial cell, if there is sufficient calcium and carbonate ions concentration in the solution (Equations 6, 7) (Qian, Wang, Cheng & Wang, 2010).
Figure 1, shows the role of bacteria with ureasic activity in calcium carbonate precipitation and the ATP generation system in this process.
In section (1), urea diffuses into the bacterial cell according to the concentration gradient, in (2) urea hydrolysis causes an increase in pH due to ammonia production, in (3) ammonia diffuses out of the cell according to the concentration gradient, increasing the membrane potential Δψ, and in (4) this increase leads to a proton driving force that allows ATP generation (Wiffin, 2004).
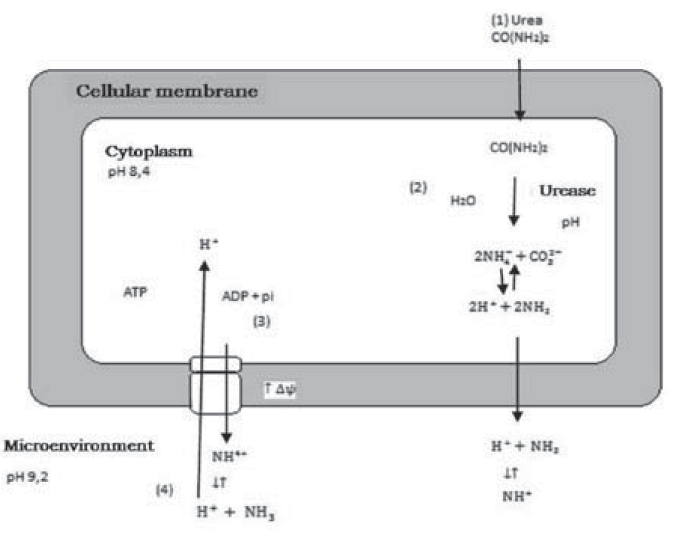
Figure 1 Role of bacteria with ureasic activity in calcium carbonate precipitation according to Wiffin (2004).
Calcium carbonate crystals
In MICP, different anhydrous CaCO3 crystals can be produced such as calcite, aragonite and vaterite, as well as other crystalline hydrated phases such as monohydrocalcite and hexahydrocalcite, and also amorphous calcium carbonate (Hammes et al., 2003a; Hammes, Seka, De Knijf & Verstraete, 2003c). The crystals more frequently obtained are calcite and vaterite (Dhami et al., 2013b; Jimenez-Lopez et al., 2008), being the latter a meta-stable and transitional phase crystal during calcite formation (Tourney & Ngwenya, 2009), which is the most thermodynamically stable phase and the main MICP product (Okwadha & Li, 2010; Stocks-Fischer et al., 1999b).
In 1999, Stocks-Fischer et al. (1999a) examined the physical and biochemical properties of calcium carbonate precipitation induced by S. pasteurii and established that the crystals formed were calcite. Chen, Okudan & Riley (2010) found that CaCO3 produced by Proteus mirabilis had an unusual morphology and structure, mainly formed by vaterite spheres. CaCO3 precipitation by a mixture of Ca2+ and CO3 -2 involves three steps as follows: 1) amorphous calcium carbonate formation with low stability and solubility, 2) their transformation into vaterite, and 3) obtaining calcite which is the most stable solid state of CaCO3 (Hua, Deng, Thornton, Yang & Amonette, 2007; Shen et al., 2006; Spanos & Koutsoukos, 1998; Wei, Shen, Zhao, Wang & Xu, 2003).
Various authors established that calcium sources induce crystal formation of different sizes. Calcium chloride induces the formation of rhombohedral crystals (DeYoreo & Vekilov, 2003; Favre, Christ & Pierre, 2009; Gorospe et al., 2013), calcium acetate induces lamellar crystals composed of vaterite (Gorospe, et al., 2013), and lactate and calcium gluconate induces more complex forms with spherical-shaped vaterite (Tai & Chen, 1998). In 2012, Abo-El-Enein, Ali, Talkhan & Abdel-Gawwad, evaluated the calcium source for the biocementation process, concluding that calcite size, morphology and degree of crystallization produced by biocementation depends on the source of calcium used; the authors obtained better results in terms of a compressive strength increase and water absorption decrease with calcium chloride, followed by calcium acetate and finally calcium nitrate. These results were corroborated by Kumari et al. (2014) in another study with calcite obtained from Bacillus sp. CR2. Wiffin (2004) showed however, that calcium nitrate added at a concentration of 2 M inhibited completely the enzymatic urease activity of S. pasteurii. Moreover, Zhang, Guo & Cheng (2015), assessed the role of calcium sources in the strength and microstructure of biocement, finding that tensile strength and compression resistance of biocement treated with calcium acetate was twice as good as with nitrate and calcium chloride. In addition, the mercury intrusion porosimetry analysis also showed that pore size distribution in samples in which acetate was used, was more uniform compared to others. Aragonite crystals with an acicular mineral morphology were detected in biocement treated with acetate, in addition to calcite and vaterite; in this study, the authors concluded that calcium acetate was the best source of calcium when used for biocementation, since it showed better performance in the tests carried out. Moreover, morphological differences of the crystals can be specific for each strain and urease activity (Hammes et al., 2003a; Park, Park, Chun, Kim, & Ghim, 2010).
Alternatively, these differences can be expressed in extracellular polymeric substance proteins produced by different types of bacteria that control calcite, vaterite or aragonite crystals formation (Kawaguchi & Decho, 2002); this occurs because these can specifically bind Ca2+ and promote carbonate precipitation (Dhami et al., 2013b). Furthermore, the composition of the culture medium can also affect crystal morphology because different species are able to precipitate varying amounts, sizes and types of carbonate crystals from the same synthetic medium (Dhami et al., 2013b, Hammes et al., 2002).
Factors that affect MICP efficiency
There are several factors that influence urease activity and amount of CaCO3 precipitated as the type of bacteria, bacterial cell concentrations, pH, temperature and calcium and urea concentrations (Al Qabany, Soga & Santamarina, 2012; Hammes et al., 2002; Mortensen, Haber, Dejong, Caslake, & Nelson, 2011).
Type of bacteria
The type of bacteria is essential for urease production, and therefore, many bacteria with ureasic activity have been studied; however, strains of the Bacillus group are the most commonly used in MICP. For example, S. pasteurii has been used for remediation, heavy metal contamination, concrete remediation and soil improvement (Li, Chen & Burne, 2013; Phillips et al., 2013b; Whiffin, van Paassen & Harkes, 2007), while B. megaterium has been used to improve concrete hardness and construction material durability (Dhami et al., 2014; Soon, Lee, Khun, & Ling, 2013).
Isolation of bacteria with urease activity
One of the main objectives of the MICP technology is to isolate and select bacterial strains that have high urease activity and that resist extreme conditions (Zhu et al., 2016a). Although in the biocementation field, as S. pasteurii is very efficient and widely used for calcite production, there are not many studies involving strain isolation. Nevertheless, there are several researchers that have isolated microorganisms from different sources. For example, Al-Thawadi (2008) isolated a strain that was patented due to its high urease activity, reaching 28 mM of hydrolyzed urea. min-1 from agricultural soils, stables and sewage sludge (exact sources are not specified). In another study, 12 strains of bacteria with urease activity were isolated from soil samples, landfills, gardens, cement and calcareous residues from a biocatalytic calcification reactor. Subsequently, when carrying out 16S rRNA sequencing studies results showed that isolates were phylogenetically related to the Bacillus sphaericus group. The authors point out that strain specificity is mainly due to differences in urease expression and calcium response (Hammes et al., 2003b). On the other hand, calcite formation was compared using bacteria with urease activity isolated from a Canadian oil field and with a commercially acquired urease enzyme; results showed that with the first method a concentration of 21.5 g.L-1 of CaCO3 was obtained, and with urease 58 g.L-1 was attained. The culture was not sensitive to temperature, which was the case with urease, but the amount of calcite produced was greater with the latter, since the high amount of urea and calcium chloride inhibited the microorganisms.
Nevertheless, using biocementation in water reservoir walls has attained positive results, as 65% porosity reduction was achieved with the culture compared to the one obtained with the pure enzyme (62%); this indicates that biomass plays an important role in pore plugging (Nemati, Greene & Voordouw, 2005).
In another study conducted by Al-Thawadi (2008) in Perth (Australia) bacteria with high urease activity were isolated from sources such as sewage sludge and soils for the production of biocement; the authors found six strains with urease activity ranging between 11-28 mM hydrolyzed urea.min-1; when the authors optimized culture conditions, they concluded that an increase in yeast extract from 10 to 20 g.L-1 and adding nickel ions at a concentration of 10 μ M increased ureasic activity of microorganisms. Moreover, another study carried out by Jimenez-Lopez et al. (2008) found that consolidation of small limestone stones (a mineral composed mainly by calcium carbonate used to manufacture cement) was increased by calcite bioprecipitation; this research was carried out to evaluate its applicability in monument restoration procedures, activating the microbiota found in the material and without inoculating a specific strain. Thereupon, four bacterial strains found in cement were identified: Sporosarcina soli KNUC401, Bacillus massiliensis KNUC402, Arthrobacter crystallopoietes KNUC403 and Lysinibacillus fusiformis KNUC404, of which KNUC403 showed the highest compressive strength (25.6 MPa at 28 days) compared to S. pasteurii (19.4 MPa) in a mixture with cement, sand and using a phosphate buffer as control (Park et al., 2010). Moreover, a Bacillus niabensis LH1 strain was isolated from soil using soy roots and a mutagenesis method with ultraviolet light to modify microorganisms and increase enzymatic activity; the conclusion was that urea degradation depends on its initial concentration. Moreover, it was found that the mutant strain LHUM107 had a good genomic stability and exhibited efficiency in urea degradation, i.e. 92.2 % up to the 21st generation (Li, Song, Li, He & Song, 2014). Likewise, Bacillus megaterium SS3 isolated from calcareous soil was applied as a biosealant in green construction materials (soil-cement blocks) reaching in the material, a 40 % reduction in water absorption, 31 % decrease in porosity, and 18% increase in compressive strength (Dhami et al., 2013b).
Concentration of bacterial cells
It is necessary to determine the optimal cellular concentration for a biocementation process, establishing the specific time in which bacteria are in their exponential phase and where the highest enzyme production occurs for the strain of interest, in order to optimize calcium carbonate production conditions. This must be established because high bacterial cell concentrations (from 106 to 108 cells) increase the amount of calcium carbonate precipitated by MICP, increasing also the concentration of urease for urea hydrolysis (Okwadha & Li, 2010). Thence, urea hydrolysis is directly related to bacterial biomass that has an even greater influence than the initial urea concentration. Stocks-Fischer et al., (1999b) stated that bacteria acted as nucleation sites in calcite precipitation; in addition, they compared MICP efficiency with chemical precipitation induced at pH 9.0 and confirmed that 98 % of the calcium concentration was microbiologically precipitated in water, while only 35 % was chemically obtained. This difference occurred because bacteria provide nucleation sites for CaCO3 and create an alkaline environment for calcite crystals growth induction.
pH
Calcite precipitation is influenced by pH because urease is only activated at a specific pH where urea hydrolysis occurs. MICP is carried out at a pH between 7.0 and 9.5 (Dupraz et al., 2009; Ferris et al., 2004; Stocks-Fischer et al., 1999b). However, it is necessary to optimize the pH range for MICP for any specific bacteria strain that will be used. For example, a study conducted by Annamalai, Arunachalam & Sathyanarayanan (2012) using S. pasteurii NCIM 2477 optimized pH conditions for the culture used to produce calcium carbonate crystals, and they concluded that the appropriate pH was 7.5; however, although a pH of 8.0 has also been reported as adequate for S. pasteurii ATCC 6453 and KCTC 3558, a higher pH decreases enzymatic activity (Gorospe et al., 2013; Stocks-Fischer et al., 1999b). Moreover, if pH levels are very low, carbonate tends to dissolve and it is not precipitated. In 1999, Stocks-Fischer et al. (1999a) examined physical and biochemical properties of calcium carbonate precipitation induced by S. pasteurii, and from their kinetic results, they found that enzymatic activity was significantly high at pH 7.7, where calcite precipitation was favored.
Temperature
Temperature range for enzymatic hydrolysis is wide varying with the species, but enzymatic urea hydrolysis is temperature dependent, ranging from 20 to 37 ° C (Al-Thawadi, 2008; Mitchell & Ferris, 2005). Mitchell and Ferris (2005) reported that urease activity increases 5 to 10 times when temperature increases 15 to 20 °C, and 10 to 20 °C, respectively. Dhami et al. (2014) found that urease is completely stable at 35 °C, but when the temperature rose to 55 °C its enzymatic activity was reduced to 47 %. Williams, Kirisits & Ferron (2016) showed that urea viability and hydrolysis are affected at extreme temperature and pH conditions (i.e. 55°C and 13.6, respectively), but this impact is reduced to 45°C and pH of 12.9.
Urea and calcium concentrations
According to Mobley & Hausinger (1989), microorganisms use urea as a source of nitrogen and energy as shown in Figure 1, from where they obtain ATP for their physiological processes; however, these processes also produces ammonium and carbonate, and if calcium ions are present, calcium carbonate precipitates (Stocks-Fischer et al., 1999a), as the surface of bacteria is negatively charged (Williams et al., 2016) and facilitates the adhesion of Ca2+ acting as nucleation sites. Therefore, concentrations and sources of calcium ions are relevant for MICP processes (Okwadha & Li, 2010), even more than urea concentrations. Moreover, essays using S. pasteurii ATCC 11859 confirmed that when calcium and urea concentrations are high (above 0.5 M), the calcite precipitation efficiency is reduced, and on the contrary, with low amounts of these compounds (0.05 to 0.25 M) this efficiency is increased.
De Muynck, De Belie & Verstraete (2010a), reported that the best urea and calcium chloride concentration for Bacillus sphaericus LMG 225 57 is 0.5 M and 0.25 M, respectively. Onal-Okay & Frigi-Rodrigues (2014), optimized calcium carbonate production from S. pasteurii ATCC 11859 using the response surface methodology, and found that the optimal urea, calcium chloride and nickel nitrate concentrations were 42.12, 6.93 and 0.071 g.L-1, respectively; moreover, under these conditions an enzymatic activity of 3.4 U.mL-1 had achieved, i.e. 2.5 times higher than the one found in literature. In another research conducted with Proteus vulgaris, results showed that when calcium concentration was 250 mM calcite precipitation increased by 100%, regardless of the initial urea concentration; therefore, optimal conditions were achieved with 666 mM of urea, 250 mM of calcium ions and a bacterial concentration of cells.mL-1 (Okwadha & Li, 2010). Moreover, with high concentrations of urea (20 and 50 g.L-1) and calcium chloride (30 and 75 g.L-1) it was demonstrated that calcite enzymatic activity and production in bacteria are inhibited (Nemati et al., 2005).
Sources of nutrients
For microorganism growth, Abo-El Enein et al. (2012) , used as an enrichment medium to isolate bacteria with urease activity, yeast extract, ammonium chloride, potassium sulfate and urea, and incubating at 28 °C for 36 hours and at a shaking frequency of 130 rpm. Then, it was cultured in nutritious agar plates with 8% urea, carrying out a phenolphthalein test to confirm pH increase that indicates presence of urease activity. Instead, Achal, Mukerjee & Sudhakara Reddy (2013) used a nutritive broth with 2 % urea, 25 mM calcium chloride and a pH of 8, incubating at 37 °C and at a shaking frequency of 130 rpm.
On the other hand, alternatives have also been used to replace nutrients such as Torula yeast ( Cyberlindnera jadinii), brewery waste yeast, Vegemite® and acetate (which lowered production costs by 95 % achieving a level of ureasic activity of 21 mM hydrolyzed urea.min-1) (Wiffin, 2004), fermented maize liquor (obtaining higher calcium carbonate concentrations and lower water penetration values) (Achal, Mukherjee & Reddy, 2011a), and lentils (zhu et al., 2016b). Although it is still necessary to implement the use of available economical and agroindustrial waste to reduce culture medium costs.
MICP Applications
Calcium carbonate precipitation induced microbiologically is an effective process and an environmentally friendly technology that can be applied to solve problems such as heavy metal contamination, soil and sand bioconsolidation, biocement, CO2 sequestration, among others (Achal & Mukherjee, 2015a; Phillips et al., 2013a; De Muynck et al., 2010a).
Heavy metal removal
Heavy metals are non-degradable chemical species and are considered stable and persistent pollutants when they are deposited in the environment; in addition, living beings are unable to metabolize these, generating contamination by bioaccumulation within the food chain (Mancera & Álvarez, 2006), which causes alterations in different ecosystems, and reducing the life quality of different organisms (Déniz, Díaz, Ramón Sánchez & Trujillo, 2010; Madero & Marrugo, 2011). The World Health Organization (WHO) and the Food and Agricultural Organization of the United Nations (FAO) recognize the vulnerability of water resources in places where heavy metal contamination persists (WHO, 2006).
Nonetheless, biocement can be applied in the removal of heavy metals, involving the formation of calcite with the subsequent incorporation of heavy metals in the crystal structure; here, these would be embedded or retained and costs would be reduced in the purchase of bases or hydroxides that are generally used to precipitate these (I§ik et al., 2012, Li et al., 2013; Nemati & Voordouw, 2003; Warren, Maurice, Parmar & Ferris, 2001). With biocementation, between 88 and 99 % nickel, copper, lead, cobalt, zinc and cadmium has been removed using S. pasteurii ATCC 11859 after a 48-hour incubation period and finally, metals were deposited around the cell cover in rhombohedral, spherical and needle-shaped crystals (Li, Cheng & Guo, 2013). Likewise, the effect of urea on the precipitation of calcium ions as calcite in synthetic water was also evaluated, and the effective urea concentration for this process was established in 15 mM. The authors highlighted the importance of this new technology applied in the removal of calcium ions that clog pipes, boilers and exchangers leading to a malfunction of industrial equipment (I§ik et al., 2012).
On top of this, the relationship between heavy metals and resistance to antibiotics regarding bacteria with urease activity isolated from abandoned soils and mines was studied, and results showed that heavy metal resistance of these isolates was associated with resistance to antibiotics. In addition, immobilization of these metals in isolates was examined through calcium carbonate precipitation induced microbiologically; thus, cylinders treated with bacteria, a resistance 3.7 times higher than the control, plus heavy metals immobilization was observed when analyzing the biocement elaborated; unfortunately, no leaching studies were carried out to verify if heavy metals remained in the matrix or left it easily (Kang & So, 2016).
Other studies with strontium found that calcium carbonate precipitation is possible capturing in 24 hours up to 95 % of this metal in a solid phase (Warren et al., 2001), which was consistent with the solid phase formation corresponding to strontium carbonate. X-ray diffraction showed presence of calcite in controls, while in strontium and uranium dioxide, precipitation of both calcite and vaterite, which are calcium carbonate meta-stable polymorphs were observed. In other studies carried out by Hammes et al. (2003a, 2003b), elimination of ca. 90 % of calcium in a semi-continuous reactor and the formation of a calcifying sludge were achieved; however, the authors suggest to carry out in-depth studies regarding urea dosage, precipitation retention times and biocatalytic sludge management. Another study carried out by Ferris et al. (2004) investigated the kinetics of calcite precipitation induced by urea hydrolysis in S. pasteurii at different temperatures in artificial groundwater, and found that the low conversion of calcite in the sample indicates that magnesium and ammonium ions convey additional restrictions to the kinetics of the reaction; moreover, the authors emphasize the need for studies that simulate natural conditions, which is important in the development of in situ bioremediation processes where high performance is required.
Soil and sand bioconsolidation
In geotechnical engineering, bioconsolidation is related to the prevention and stabilization of erosion therefore, conventional techniques as addition of chemicals to improve the soil giving it greater strength and rigidity have been applied. However, these methods have consequences such as water and air pollution, and besides, these are expensive and have problems distribute these uniformly (DeJong et al., 2010).
MICP has commonly been found to be an effective technique to improve soil quality.
Authors as Wiffin et al. (2007), have used MICP to increase sand stabilization and have obtained a decrease in porosity and an increase in soil hardness. Likewise, DeJong et al. (2010), Gorospe et al. (2013), and Mortensen et al. (2011), have stated that the induction of calcium carbonate precipitation binds sand grains, increasing soil strength and stiffness. Ivanov and Chu (2008) compared the cost of traditional chemical glue with microbial binding, and concluded that the latter is cheaper. The chemical technique is not only expensive but requires many injection vessels for the treatment of large volumes; however, by using MICP, reagents and catalysts can be transported to where they are required (Dhami et al., 2013). Canakciet, Sidik & Halil Kilic (2015), evaluated the biocementation process in sandy organic soils and found that calcite precipitation increased by 20 % in treated samples, influencing the compressibility and cutting force of the sample used.
Moreover, Wiffin (2004), evaluated the effectiveness of biocementation using two sandy soils and achieving a hardness eight times higher compared to the control material. Many researchers have however, reported the improvement of hardness and the reduction of soil permeability using bacteria with urease activity (Carmona, Oliveira & Lemos, 2016; Chu, Stabnikov & Ivanov, 2012; DeJong, Fritzges & Nüsslein, 2006; Ferris et al., 1996; Whiffin et al., 2007).
Biocementation or biocement preparation
An indication of the prosperity and development of the world is the construction of roads, buildings, bridges, among others. Therefore, the use of materials for this purpose has exploded in recent years and will continue to grow in the future. Cement stands out as the most used artificial material in the world (Achal & Mukherjee, 2015b), producing annually ca. 4.6 billion tons (CEMBUREAU, 2016), and specifically in Colombia, gray cement shipments increased to 12,101 tons in 2016 (DANE, 2016). However, its production is responsible for approximately 6% of current CO2 emissions, and specifically the construction industry worldwide reaches ca. 50% of total emissions (Achal et al., 2015); therefore, it is necessary to look for sustainable alternatives in terms of construction materials.
An alternative for cement and chemical glues is biocement (De Muynck et al., 2010a) as it can join materials using MICP to seal fractures and improve hardness and durability of various materials (Dhami et al., 2014; Phillips et al., 2013b). Moreover, it has been possible to reduce water absorption from 65 to 90 %, providing an increase in freezing and thawing resistance, which is extremely relevant to reduce degradation of the material (De Muynck, Debrouwer, De Belie & Verstraete, 2008b).
Pure strains and mixtures of bacteria with urease activity have been studied in surface treatments of cement and concrete, finding better results with pure strains, i.e. achieving a reduction in capillarity, water absorption and gas permeability at surface level; albeit, results were similar to those obtained with traditional water repellents such as xylans and xylosans (De Muynck, Cox, De Belie & Verstraete, 2008).
Similarly, the application of spores of Bacillus pseudofrmus DSM 8715 and B. cohnii DSM 6307 has been tested directly on the cement, proving that they remain viable for four months, and although a decrease in the pore size during the adjustment of the stone and cement limited the useful life of the spores, these were suggested more as potential self-sealing agents (Jonkers, Thijssen, Muyzer, Copuroglu & Schlangen, 2010).
Inclusion of other materials in the biocement
Various materials have been tested to elaborate biocement, highlighting: cement mixtures of the Portland type and sand (Abo-El-Enein et al., 2012; Abo-El-Enein, Ali, Talkhan & Abdel-Gawwad, 2013; Park et al., 2010), ash (Achal, Pan, & Õzyurt, 2011b), river sand and cement in a 1:3 ratio (Achal et al., 2013) and sand (Annamalai et al., 2012). The culture is generally added to the materials in a column so that when calcium carbonate precipitates, important factors that are taken into account in the durability of cement can be assessed, such as: water absorption (Abo-El-Enein et al., 2012, 2013; Achal et al., 2011), compression resistance (Abo-El-Enein et al., 2012, 2013; Achal et al., 2011a), crack or fissure remediation (Achal et al., 2013), impermeability to water by measuring penetration resistance (Achal et al., 2011a) and total porosity (Achal et al., 2013); the results obtained were positive, including a porosity reduction of up to 50% in the materials used, and with a significant decrease in chlorine and water permeability, which would allow their potential use in structures or buildings. However, the inclusion of other materials such as waste ash from a thermoelectric plant (Achal et al., 2011a), ashes and waste from the silica industry (Chahal & Siddique, 2013), rice husk ashes (Siddique et al., 2016), solid industrial waste such as cement and lime kiln dust (Cuzman, Rescic, Richter, Wittig & Tiano, 2015) have also been assessed.
CO 2 sequestration
MICP has attracted the attention of researchers due to its use for CO2 sequestration as carbonate minerals such as calcite, magnesite, and dolomite (Seifritz, 1990). In nature, carbon dioxide is sequestered by chemical fixation into carbonates, but the reaction is very slow (Dhami et al., 2013b). Therefore, researchers have seen the possibility of using the biological pathway through carbonic anhydrase which is a metallo-enzyme that contains zinc and that catalyzes the reverse reaction of CO2 hydration to bicarbonate in prokaryotes and eukaryotes. This method is much safer and user-friendly with the environment than conventional methods for sequestration of environmental carbon dioxide. Furthermore, it has been shown that carbonic anhydrase of Bacillus megaterium acts synergistically with urease in carbonates production (Dhami et al., 2014). Bond, Stringer, Brandvold, Simsek & Egeland (2001) reported the transformation of CO2 into bicarbonate in the presence of Ca2+ ions in artificial seawater, and observed a rapid decrease in carbon dioxide concentration and an increase in calcium carbonate synthesis with carbonic anhydrase.
Advantages and Limitations of MICP
Among the MICP advantages we find: 1) retention of evident permeability by water absorption registered in biocemented surfaces (Tiano, Biagiotti & Mastromei, 1999); 2) cost reduction in the process since the enzyme can be reused several times (Wiffin, 2004); 3) pore reduction with direct use of microbial culture without needing to concentrate cells or extract enzyme, with no additional processes are required (Al-Thawadi, 2008); 4) bacterial source is more resistant to biocementation conditions than enzymes extracted from plants (Wiffin, 2004).
On the other hand, within MICP limitations we find: 1) production of ammonia that can be toxic and a risk to human health in high concentrations (Harkes, van Paassen, Booster, Whiffin & van Loosdrecht, 2010); 2) microbial processes are slow and are usually more complex than the chemical one, because the microbial activity is dependent on factors such as temperature, pH, concentration and diffusion rates of nutrients and metabolites (Ivanov & Chu, 2008); and 3) economic limitations in the acquisition of nutrients during field application. Therefore, identification of various types and low-cost food sources are required for its application in MICP.
Calcium carbonate precipitation induced microbiologically is related to calcium carbonate formation due to the presence of microorganisms and their physiological processes, where several metabolic products are secreted that react with calcium ions from the culture medium leading to the formation of CaCO3.
This process can be carried out by several microorganisms, especially by bacteria of the Bacillus genus, which have demonstrated high urease activity.
The efficiency of calcium carbonate production can be affected by the type of bacteria, concentration of bacterial cells, pH, temperature and amount of calcium and urea added to the medium. MICP applications are diverse and include heavy metals removal, soil bioconsolidation, elaboration of materials such as biocement, and sequestration of carbon dioxide.