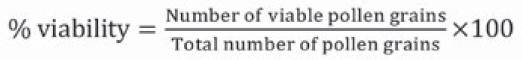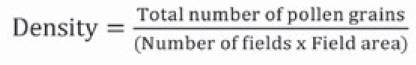Introduction
Phaseolus acutifolius A. Gray, commonly known as tepary bean, is a species native to northern Mexico and southwestern United States, and is a promising species in the face of climate change (Blair, Pantoja & Muñoz, 2012; Rao, Beebe, Polania, Ricaurte, Cajiao, Garcia & Rivera, 2013). Since this legume has features of great interest such as stress tolerance to drought, high temperatures and salinity, as well as resistance to some diseases and pest insects (Muñoz, Duque, Debouck & Blair, 2006). These traits highlight this species, not only as a potential donor of resistance genes to common bean P. vulgaris, but also as a genetic resource with great agricultural value per se (Muñoz, Blair, Duque, Tohme & Roca, 2004).
However, P. acutifolius has a low genetic variability (Muñoz et al., 2006), and even more in its cultivated forms, probably due to a bottleneck effect when domestication took place from a small number of species populations (Jiménez & Acosta, 2012).
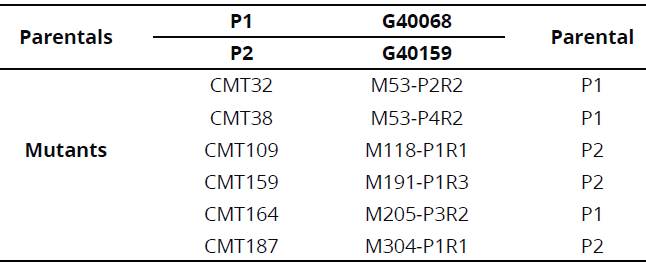
To increase genetic variability in tepary beans, a program for the induction of mutations with the chemical agent ethyl methanesulfonate (EMS) was initiated within the framework of the project "Heat effects on tepary bean and its relatives" financed by the International Atomic Energy Agency (IAEA), Vienna, Austria. Mutant lines originated from two cultivated accessions of tepary bean, G40068 and G40159, which were selected for their stress tolerance traits to drought and heat (Rao et al., 2013). Moreover, mutated plants were reproduced by self-pollination (no crosses were carried out), until the fifth generation (M5) was obtained.
In this sense, an important variable to evaluate is the pollen viability, since pollination and fertilization are fundamental traits for plant breeding processes and crop production (Frankel & Galun, 1977), and failures in pollen formation and development are main causes of non-viability of species or hybrids (Lagos, Caetano, Vallejo, Muñoz, Criollo & Olaya, 2005). In addition, the evaluation of pollinic viability would allow identifying androsterile mutants with great use for cross breeding, and this in turn generates an indicator of tolerance to water stress and high temperature, related to other performance parameters.
Drought stress is one of the limiting growth and yield factors in many crops, which together with high temperatures during their growth period, generates a clear reduction in grain production; in addition, high temperatures increases physiological problems caused by water stress (Barrios, López & Kohashi-Shibata, 2011; Rao et al., 2013).
On the other hand, it is important to recognize that legumes are associated with rhizobia, which are a group of bacteria that induce nodule formation in roots, where they fix atmospheric nitrogen (Cuadrado, Rubio & Santos, 2009). The use of suitable rhizobia strains can increase crop yield, which depends on the efficiency of bacteria to fix nitrogen, their saprophytic competence against other soil microorganisms and other strains that colonize infectious points in roots (Mora, 1995). The use of Bradyrhizobium sp. is justified when considering that some strains have grown tolerant to temperatures of 40 °C and at pH of 11 (Cuadrado et al., 2009), which is favorable under high temperature conditions.
Taking into account these factors, the aim of this study was to determine the effect of lines, inoculation with rhizobia and water stress on the viability and amount of pollen of the M5 mutant lines of P. acutifolius and its parental genotypes.
Material and methods
Plant material
Plant material used to evaluate the effect of high temperature stress under irrigation and drought conditions were used. The trial was conducted under greenhouse conditions at the International Center for Tropical Agriculture (CIAT) in Palmira, Valle del Cauca, Colombia. We evaluated 123 plants of six mutant tepary bean lines of the M5 generation: CMT32, CMT38, CMT109, CMT159, CMT164 and CMT 187 and their parental genotypes G40068 and G40159 (Table 1). Between 25 and 35 days after planting, two fully closed floral buds from the middle third were collected from each plant and stored at 4°C in jars with a 3:1 ethanol and acetic acid relation fixative solution.
Treatments
Trials were carried out in a greenhouse with high temperature conditions, controlled with Pollen viability of Tepary bean (Phaseolus acutifolius A. Gray.) mutant lines under water stress conditions and inoculation with rhizobia heaters and thermostats that prevented night temperature from falling below 25 °C. Irrigation factors and Rhizobium inoculum were applied in a subdivided plot design, where the main plot was irrigation treatments; in this plot, two humidity conditions were evaluated: 1) pots were maintained with an irrigation of 80% field capacity throughout the evaluation period (irrigation); 2) pots were maintained at 40% field capacity from the twelfth day after sowing, i.e. water stress treatment (drought).
Inoculums of two strains of rhizobia were the subplots: one of Bradyrhizobium sp. (CIAT461) of slow growth, and another of Rhizobium tropici (CIAT899) of rapid growth, in addition to the control without inoculum. The sub-subplots correspond to the lines evaluated (mutant lines M53P2R2, M53P4R2, M118, M191, M205 and M304 and the parental genotypes G40068 and G40159).
Agro-morpho-physiological parameters
Evaluation of agro-morpho-physological parameters was carried out according to Polanía, Rao, Mejía, Beebe & Cajiao (2012). The physiological measurements were made during the half-fill period, including variables such as relative leaf chlorophyll content (SPAD units) measured with the SPAD-502 Chlorophyll meter, stomatal conductance (mmol.m-2.s-1) measured with a Decagon SC-1 porometer, and temperature of the plant crown was measured with a Telatemp AG-42D infrared thermometer. Variables associated with production, morphology and nodulation parameters were obtained during the postharvest period. Values as seed weight, number of pods, number of seeds, number of seeds per pod, weight of 100 seeds, weight of pods, stems and roots as well as number of nodules and their weight were measured for each plant.
Plate assembly
Plate assembly was carried out in the molecular biology laboratory of Universidad Nacional de Colombia, Palmira Campus. Then, flower buds were dissected to extract the keel that was macerated with a pick in 200 μl tubes with 15 μl acetic carmine at 2 %, and later a dilution of the pollen grain suspension was made adding 100 μl of distilled water. The suspension was served on slide plates and dried in an oven at 50°C, after which, permanent plates were prepared and these were sealed with resin and coverslips. In this way, standardized plate lots were obtained that were later photographed and analyzed in blocks.
Additionally, a pollination tube germination test was carried out with five fresh pollen samples that were not yet fixated, with a culture medium containing 100 ppm of H3BO3, 250 ppm of Ca(NO3)2, 100 ppm of MgCl2, 100 ppm de KNO3, 200 ppm de CaSO4 and 28% of sucrose at a pH of 7.2 (Vásquez, White & Roca, 1990). This was done to verify the good state of the pollen with direct evidence on its viability.
Images
Observations and photographs were carried out in the electronic microscopy laboratory of Universidad Nacional de Colombia, Palmira Campus. Plates were observed in an Axio Lab A1 Carl Zeiss microscope, and digital photographs were taken with an Axio Cam camera coupled using the ZEN software. Stained pollen was observed and a photograph of nine sequential optical fields was made with a 3x3 grid, which covered most of the visible pollen on the plate; this was carried out using a 5x objective, and in this way, each microphotograph covered an approximate area of 5 mm2.
Image analysis
When series of digital pollen photographs were obtained from all plant materials, counting of viable and non-viable pollen grains present in the complete optical fields was carried out, until a minimum of 500 pollen grains per floral bud were counted. Pollen count was done manually, assisted by the plugin Cell Counter of the ImageJ software (Schneider, Rasband & Eliceiri, 2012). With this method, dyed pollen grains were considered as viable and those that were not dyed were deemed as non-viable (Porch & Jahn, 2001). Raw data were tabulated in a matrix, from which viability and density indicators were obtained with Equations 1 and 2.
Pollen density value is a parameter of the amount of pollen grains per square millimeter (PG.mm-2) in the plaque preparation, and this has been proposed in this work as an indicator of pollen production by floral bud.
Results
Assembling the plate and macerating a keel in tubes was an efficient practice for the massive evaluation of pollen grain viability, as with this technique cleaner plates were obtained, i.e. without too much tissue, than using individual anther dissection plates, and with no apparent damage to pollen grains. Carmine stain allowed differentiating well-stained viable pollen grains from non-viable pollen grains that were observed as unstained (Figure 1). Manual pollen count with the assistance of the ImageJ software was comfortable and efficient, allowing counting 500 pollen grains in approximately 12 minutes.
Furthermore, pollen germination test worked correctly in plant material evaluated, allowing the generation of a prominent pollen tube in multiple pollen grains (Figure 1c), However, as plant material evaluated for this test was limited, this data has not been considered in this study and only represents a testimonial report of the effectiveness of the culture medium used, and the correct germination of the pollen tube in pollen grains.
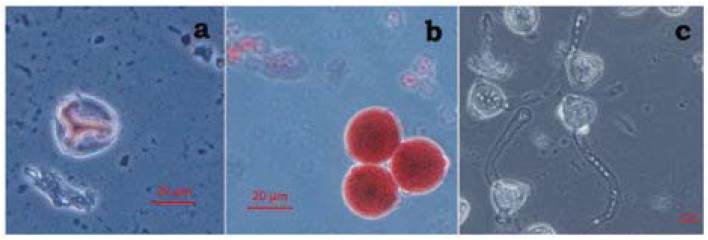
Figure 1 Microphotographs of a. (40X) Non-viable pollen from CMT38 (M53P4R2, CIAT461, drought) b. (40X) Viable pollen from CMT136 (G40159, without inoculum, irrigation) c. (20X) Pollinic tube growing (G40068, CIAT461, drought)
Regarding average percentage of pollen viability in all treatments evaluated, values obtained were higher than 90 % (Figures 2a and 2b). On the other hand, pollen density showed average values between 20 and 40 PG.mm-2 among treatments and genotypes (Figures 2c and 2d). Individual cases were identified where density was less than 6 PG.mm-2 (M53P4R2, without inoculum, and under drought conditions) or was higher than 100 PG.mm-2 (G40068, CIAT899, under drought conditions).
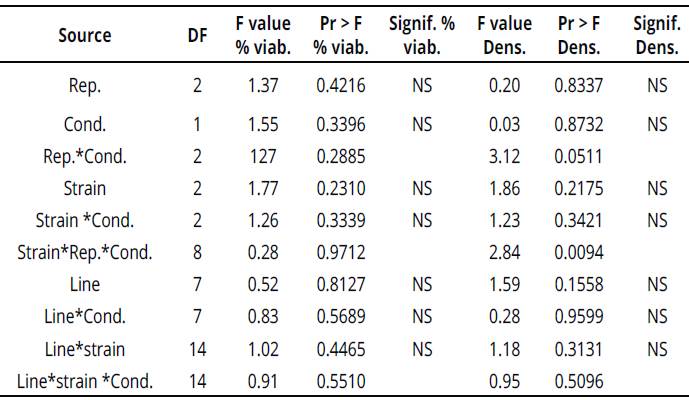
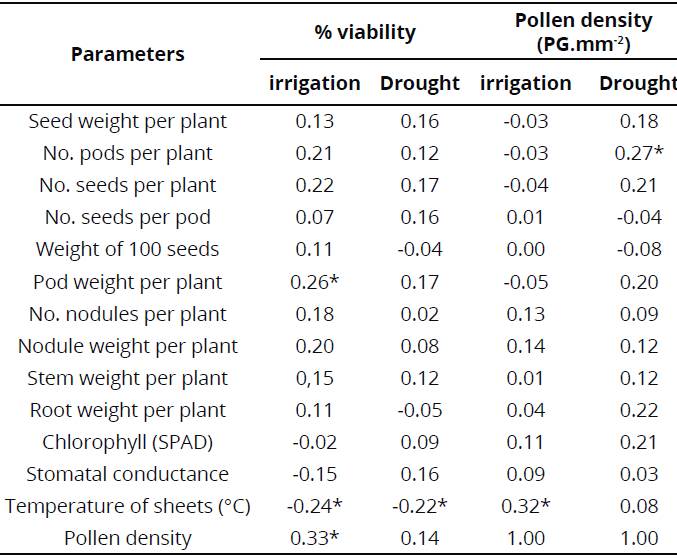
Nevertheless, when carrying out an analysis of variance, results indicated that there are no significant differences among any of the conditions evaluated in treatments (Table 2); this suggests that there was no effect of treatments (moisture condition, strain and genotype) on pollen viability and density percentage. Additionally, Pearson's correlation analysis showed that there is a positive correlation between pollen densities during water stress conditions (drought), with number of pods; moreover, this was similar to the correlation found for viability percentage in irrigation with pod weight.
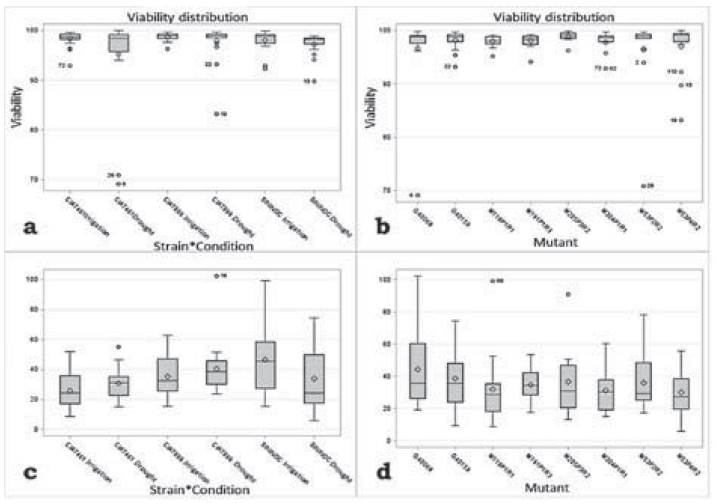
Figure 2 Diagram boxes distribution of viability percentages according to: a. strain x humidity condition, and b. genotype; and distribution of pollen density (No. pollen.mm-2) according to: c. strain x humidity condition, and d. genotype.
However, viability percentage and pollen density were positively correlated when measured under irrigation conditions (Table 3); this could indicate that, in the irrigation treatment, the two viability parameters evaluated responded in a similar way, and that a plant with a high viability percentage also has a high pollen production.
Discussion
We found that the use of photographs is a useful alternative for evaluating pollen viability, since it allows having a digitalized record of the state of the samples at the time the photograph was taken. Moreover, the analysis becomes easier when using the Image software (Schneider et al., 2012) that streamlines the processing of a large number of pollen samples, through a safe manual count, minimizing counting errors in the microscope.
Drought and high temperature stress are the most common factors that affect pollen viability in a large number of species during their development in the anther (Porch & Jahn, 2001).
However, the high percentage of pollinic viability in the tepary bean genotypes evaluated, is an important indicator of the degree of tolerance of the species to high temperature stress, especially at night temperatures above 25 °C, where the susceptibility is higher for many plants (Porch & Jahn, 2001).
Moreover, even with two moisture condition treatments, viability percentage values were always high, e.g. the average viability percentage with irrigation was of 98.37 %, while the one with drought was of 96.85 %. However, it is possible that morphological pollen staining could have overestimated the percentage of pollen viability as has already been reported in other works (González, Estévez, Castillo, Salomón, Moré & Hernandez, 2002); in this sense, readings of such a high viability in all treatments could have been biased, and there were normally variations, as has been reported in other legumes, where pollen germination percentage was reduced by heat stress (Jiang, Lahlali, Karunakaran, Kumar, Davis & Bueckert, 2015). However, it should be noted that this technique was used because it allowed the preservation of the original pollen grain structure (from the time they were collected) in the fixative solution, before these were processed for analysis; therefore, we also thought of estimating an additional pollen production indicator.
For instance, pollen production measurement is a parameter that can be carried out conventionally by counting pollen grains of an anther (Prasad, Boote & Allen, 2006), but this involves making a separate assembly of plates from the ones used for pollen viability, which means spending more time, resources and effort. So, given the need to quantify an indicator for pollen production that would decrease working time and provide additional information on pollen viability status, the inclusion of a new variable such as pollen density was considered. This variable assumes that since the staining and assembly technique is standardized, the amount of pollen grains per unit area in the preparation is proportional to the production of pollen grains per floral bud (ten anthers).
Bearing this in mind, when talking about an average density range between 20 and 40 PG.mm-2 an extrapolation to pollen production can be done, e.g., assuming that all floral bud pollen is found in nine optical fields photographed (45 mm2 in total), it can be said that the production ranged from 900 to 1800 pollen grains per flower bud; however, although considering the loss of pollen grains in the plate assembly process, it is likely that the actual number was higher.
Furthermore, pollen density was a more heterogeneous variable than the viability percentage, which allows us to consider that this parameter provides additional information to an evaluation limited only to viability percentage; for example, in a M53P4R2 plant without inoculum under drought treatment, which has a viability percentage of 89 % but with a density of 5.8 PG.mm-2, this would suggest that, although most of the pollen produced was viable, it was produced in very low amounts.
However, neither the viability percentage nor the pollen density values had significant differences between treatments according to the ANOVA (Table 2), which means that none of the treatments affected the viability indicators studied.
It should be noted that, apart from the significant differences, we observed, for example, higher density values in the control without inoculum compared to treatments with inoculum (Figure 2 c and 2d); this suggests that the strains used did not influence positively pollen production.
On the other hand, the Pearson's correlation coefficients indicated that there were correlations between viability variables and some performance parameters under different conditions. The first feature is the number of pods that correlates positively with pollen density under drought condition; nonetheless, it is worth noting that when plants are under irrigation conditions, no correlation is observed.
Another value that correlates positively with the viability indicator is pod weight per plant that correlates positively with viability percentage in irrigated condition; this implies that viability values are useful under certain circumstances, to estimate performance parameters.
On the contrary, another important correlated parameter is leaf temperature that was negatively correlated with viability percentage; this indicates that at higher temperature in leaves, lower viability percentage is obtained; this could be related to mechanisms associated with changes in sugar metabolism, which implies that high levels of CO2 could indirectly protect pollen from thermal stress (Aloni, Peet, Pharr & Karni, 2001).
Conclusion
We can conclude that pollen viability in original and mutant tepary bean lines following the methodology established in this study, was not affected by any of the treatments and always remained high, i.e. that neither irrigation, rhizobia strains nor the lines evaluated influenced pollen viability values. In addition, these viability values could be related to a high degree of tolerance of the species to water stress conditions and high temperatures. On the other hand, the correlation between pollen viability measurements and other agro-morphophysiological variables was low; however, pollen density stood out as a parameter as potential pollen viability indicator, which could be considered when evaluating yield, as under water stress conditions, it correlated positively with production of pods per plant.