Introduction
Rice hoja blanca disease (RHB) is a highly important economic constraint to rice (Oryza sativa L.) production. Yield losses can reach 100% when susceptible varieties are sown and infected vectors are present in the field (Morales & Jennings, 2010). The causal agent of RHB is a Tenuivirus named Rice hoja blanca virus (RHBV). It is composed of four different filamentous particles that are 3 nm in diameter. Each particle has a different length and contains a different segment of ssRNA. Currently, this disease affects rice fields in Colombia, Costa Rica, Ecuador, Guyana, Panama, Peru, Dominican Republic, Nicaragua, and Venezuela. Disease epidemics are intermittent or cyclical, probably because of the population dynamics of its insect vector, and the interaction between the virus and the vector insects (Jennings & Pineda, 1971; Morales & Jennings, 2010). RHBV is closely related to Echinochloa hoja blanca virus (EHBV), which is transmitted by a different Tagosodes species (Madriz, De Miranda, Cabezas, Oliva, Hernandez & Espinoza, 1998). RHBV is detectable in both plants and insects through ELISA (enzyme-linked immunosorbent assay). Virus incubation requires 20 to 22 days in the insect and 7 to 9 days in 10-day-old plants (Pantoja, Fisher, Correa, Sanint & Ramírez, 1997). The virus cannot be transmitted mechanically or through seeds (Malaguti, Diaz & Angeles, 1956). The virus also causes disease in the vector insect, shortening its life and decreasing its fecundity (Jennings & Pineda, 1971).
Symptoms of RHB depend on the variety and age of the plant. Young leaves have short chlorotic stripes, parallel to the midrib. Older plant leaves lose their green color as the chlorotic stripes coalesce into wide white (or pale yellow) bands. These symptoms give the disease its name (hoja blanca means white leaf in Spanish). Eventually, the virus causes necrosis, which begins from the apical part of the leaves and extends toward the base of young plants. Plant tillering decreases, growth is poor, and, sometimes, the disease causes the death of the plant (Pantoja et al., 1997). Normally, during late infection, some tillers are also affected, and the panicles are sterile and deformed. Poor root development has also been observed under controlled greenhouse conditions (unpublished data). Unfortunately, once infection occurs, there is no chemical treatment to control or cure the disease (Pantoja et al., 1997).
Sogata is the common name of the RHBV insect vector, Tagosodes orizicolus (Müir) (Hemiptera: Delphacidae). This species is distributed in almost all of the tropical and subtropical rice-growing areas of the Americas. Sogata sucks nutrients from phloem and excretes honeydew on the leaves, which both encourages fungal growth and reduces the photosynthetic capacity of the plant. In the absence of RHBV, high insect populations cause severe crop damage or hopper burn. Sogata adults are relatively sedentary, but disperse easily with strong winds or water currents (Heinrichs, 1994). Sogata transmits RHBV persistently and the virus can be acquired either by feeding on infected plants or transovarially. Interestingly, not all Sogata are capable of virus transmission (Galvez, Thurston & Jennings, 1961). However, even when the proportion of Sogata capable of transmission is as low as 1%, serious damage results when susceptible varieties are sown (Calvert & Reyes, 1999). Moreover, both chemical and biological control are often ineffective in managing RHBV transmission in Sogata populations. Therefore, RHBV-resistant lines and cultivars are the most effective and economical means of managing hoja blanca disease in rice.
Rice hoja blanca disease resistance has proven to be an effective crop management tool. In resistant lines, most plants remain symptom-free, and ELISA fails to detect virus in the plants. "However, RHBV-resistance in the cultivated rice species O. sativa, especially among cultivars that produce the indica grain type preferred in Latin America, is rare and a major obstacle in the implementation of integrated RHB management programs" (Morales & Jennings, 2010, p.13). Thus far, a single cultivar, Fedearroz 2000, is the best source of resistance to RHB disease. Other sources of resistance to RHBV have been identified in O. sativa japonica germplasm and transferred to indica backgrounds suitable for use in Latin American breeding programs (Zeigler, Pantoja, Duque & Weber, 1994). The International Center for Tropical Agriculture (CIAT) and the Latin American Fund for Irrigated Rice (FLAR) have been evaluating thousands of lines for years. However, no sources have yet provided RHB resistance that is as effective as that of Fedearroz 2000. Thus, only a few sources of resistance have been used to produce all of the commercially available RHB-resistant cultivars. Without the discovery of new efficacious sources of resistance, rice production will be at risk when the virus breaks the resistance of Fedearroz 2000.
Here, we report the discovery of new RHB-resistance sources in a diverse indica panel of O. sativa. These new resistance sources constitute a resource that can be used to sustainably extend hoja blanca disease management throughout all of the rice-growing regions of tropical America.
Material and methods
Evaluations were performed with methodologies commonly used at CIAT (Triana, Cruz & Meneses, 2003). Indica genotypes were evaluated in four sequential experiments: (1) initial RHBV screening, (2) individual RHBV evaluation, (3) field trials, and (4) insect resistance screening. In each evaluation, Sogata insects were obtained from colonies maintained at CIAT. The RHBV-harboring colony contained insects that were fed on RHBV-infected plants and allowed to reproduce on a rice variety (Bluebonnet 50) that is susceptible to both the insect and RHBV. To determine the percentage of virulent insects (insects capable of transmitting the disease) in this colony, 200 individual nymphs were tested for virulence on separate, caged, RHB-susceptible 8-day-old seedlings. The nymphs were permitted to feed and transmit virus to the plants for 3 days. After 11 days, the number of plants displaying disease symptoms was determined and the percentage of virulent nymphs in the colony (62.3%) was extrapolated. This percentage is very high compared to the natural conditions where virulence ranges from 1% up to 5% when the risk of epidemic is severe (Pantoja et al., 1997).
The RHBV-free colony was begun using non-vector insects, insects that did not transmit disease to susceptible rice seedlings for at least three generations. This colony was maintained separately on RHBV- and insect-susceptible rice (Bluebonnet 50) and closely monitored for any signs of RHB disease symptoms.
Initial RHBV screening
To identify new sources of resistance against RHBV, 660 genotypes were evaluated in cages, under greenhouse conditions (27 °C, 80% relative humidity, and 12 daylight/12 night hours). We evaluated 295 genotypes belonging to a diverse indica rice panel from the International Rice Research Institute (Group 1) and a second set of lines of diverse origin that were sequenced in "The 3000 Rice Genomes Project" (Li et al., 2014) (Group 2). The genotypes were screened in plastic trays containing sterilized soil arranged in two randomized complete blocks, with three replications and 20 plants per replication. Each block included check varieties Fedearroz 2000 (resistant), Colombia 1 (intermediate), and Bluebonnet 50 (susceptible). Nymphs collected from the RHBV-harboring colony were used to transmit RHBV to the plants. An average of four nymphs per plant were released into each tray 18 days after planting. The nymphs were permitted to feed on the plants for 3 days and were then killed with imidacloprid insecticide. The number of plants showing disease symptoms per genotype was determined visually 40 days after infestation.
Individual RHBV evaluation
Individual evaluation was performed on genotypes that exhibited a percentage of infected plants lower than that of Fedearroz 2000 in the initial RHBV screening. The main difference in this evaluation was that the insects were not free to choose the plants on which they fed. Instead, four insects from the RHBV-harboring colony were confined together on individual caged plants. The cages were constructed of acetate tubes, covered with tulle fabric to prevent the insects from escaping and to provide aeration. Genotypes and the checks were planted in a completely randomized design with ten replications in one experiment and with 20 replicates in a second experiment. The insects were allowed to feed on the plants for 3 days and were then killed using imidacloprid insecticide. The number of plants displaying RHB symptoms was determined 40 days after infestation.
Trials under field conditions
The sources of RHBV resistance were evaluated under semi-controlled field conditions based on the CIAT screening (Zeigler, Rubiano & Pineda, 1988). This method releases RHBV-vectoring Sogata that are maintained in greenhouse colonies to ensure that RHBV incidence is high. Genotypes that exhibited a low percentage of infection in initial screening experiments were examined in randomized complete blocks with ten replications. Replications consisted of rows 0.5 m in length, with 0.15 m between rows and 100 plants per row. Rows were planted using dry seed. One row was the experimental unit. Fedearroz 2000, Colombia 1, and Bluebonnet 50 were included as checks in each block. Eighteen days after planting, an average of two insects per plant were released. Three days later, the insects were killed with imidacloprid insecticide. The insects came from an RHBV-harboring colony in which 48.6% of the insects were virulent. Forty days later, disease incidence was evaluated using a visual scale of damage with five levels (1, 3, 5, 7, and 9): Level 1, rows exhibiting less than 10% of plants with symptoms; Level 3, >10% and <30%; Level 5, >30% and <50%; Level 7, >50% and <70% and Level 9, >70% Levels 1 and 3 were classified as resistant, level 5 was classified as intermediate, and levels 7 and 9 were classified as susceptible. The data were recorded and analyzed as a percentage of incidences in which the rows of each genotype exhibited these reactions.
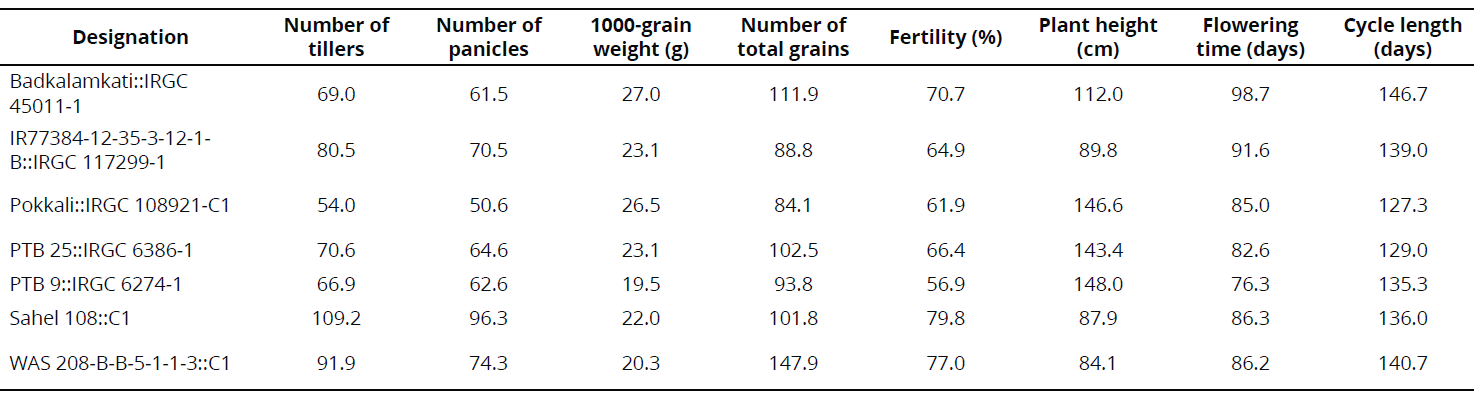
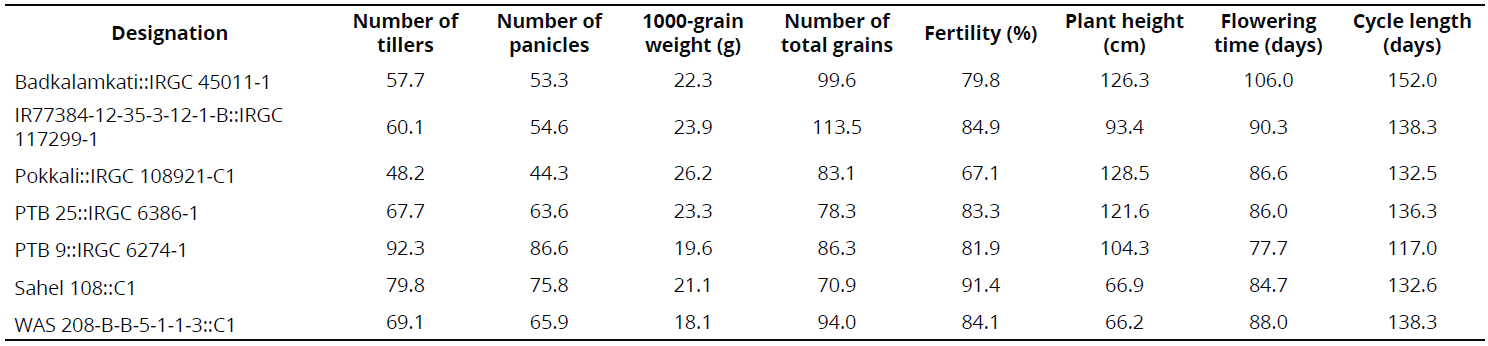
Some phenotypical traits were determined under CIAT-Palmira field conditions in 2013 and 2014 as part of a complementary study. In 2013, rows were established through transplantation, using five plants per linear meter. In 2014, the rows were established through direct seeding with 20 plants per linear meter. Data are registered in the results section in the interest of rice breeding.
Reaction to insect damage
Ideally, commercial varieties targeted for RHB-prone areas must be RHBV resistant as well as tolerant of the mechanical damage caused by T. orizicolus. As a consequence, RHB-resistance sources were tested for tolerance of the mechanical damage caused by the insects. The experimental design was completely randomized with three replications and Makalioka (resistant), Cica 8 (intermediate), and Bluebonnet 50 (susceptible) were included as checks. The experimental unit was a set of ten plants that were planted in trays with sterilized soil. Ten nymphs from the virus-free colony were released per plant 15 days after planting. The insects were allowed to feed on the plants until the time of evaluation: the day when 100% of the plants of the susceptible check died (about 8 days after infestation). Evaluation was performed using the Standard Evaluation System for Rice (www.knowledgebank.irri.org/images/docs/rice-standard-evaluation-system.pdf), in which ratings of 1 and 3 are a resistant reaction, a rating of 5 is an intermediate reaction, and ratings of 7 and 9 are susceptible reactions. The maximum value exhibited observed in a replication was reported as the final result.
Statistical analysis
Statistical analysis, including linear mixed model and least significant difference (LSD), was carried out to test the differences between the current source of resistance (Fedearroz 2000) and each one of the evaluated genotypes. The statistical analysis included a test of the hypothesis to measure the genotype effect through a linear mixed model. Adjusted means were also estimated for each genotype. In addition, a comparison test of adjusted means against Fedearroz 2000 was done through LSD method to identify genotypes better than or equal to Fedearroz 2000.
Molecular analysis and genetic variability
Population structure for the diverse indica rice panel of 295 genotypes (Group 1) was assessed. The analysis was carried out with the package Structure V2.3 using 83,374 SNPs (Rebolledo, Peña, Duitama, Cruz, Dingkuhn, Grenier & Tohme, 2016). Parameters were K=1 to 10 subpopulations, tested with 16 replications, a burning period of 20,000 iterations, and sampling period of 40,000 iterations. The final result of the population structure was obtained using the online tool Structure Plot (Ramasamy, Ramasamy, Bindroo & Naik, 2014). Based on population structure analysis, five rice genotypes showing resistance to RHBV and belonging to different subpopulations were chosen for WGS (whole-genome sequencing) at BGI Americas.
To determine whether the new resistance sources were different from Fedearroz 2000, single nucleotide polymorphisms were analyzed within and surrounding the QTL associated with RHBV resistance on chromosome 4, previously reported by Romero, Lozano, Garavito, Carabali, Triana, Villareal... & Lorieux (2014). The genomic region spanning from 2.81 Mbp to 3.8 Mbp was compared between Fedearroz 2000, the five new sources, and Colombia 1 (intermediate), Fedearroz 50 (highly antibiotic effect against the vector), and Bluebonnet 50 (RHBV-susceptible check). The whole genome sequences (WGS) of the former three genotypes were obtained from previous work (Duitama, Silva, Sanabria, Cruz, Quintero, Ballen. & Tohme, 2015); the WGS of Colombia 1 (IRGC 116970-1-1) and Bluebonnet 50 (IRGC 1799-1-1) were obtained from the 3K RGP (Li, Wang & Zeigler, 2014). The reads were analyzed using NGSEP (Next Generation Sequencing Eclipse Plugin) software with the parameters recommended in Duitama, Quintero, Cruz, Quintero, Hubmann, Foulquié-Moreno, & Tohme. (2014). Parameters used for the selection of SNPs were as follows: (1) minimum genotype quality 40, (2) SNPs genotyped in all accessions, and (3) no heterozygous SNPs. All the SNPs located in the selected region were used for the calculation of a p-distance matrix and the construction of an unrooted tree following the neighbor joining (NJ) method with the software SplitsTree4 (Huson & Bryant, 2006). Based on the SNPs obtained by WGS data, eight polymorphisms within the analyzed region of chromosome 4 were developed as Fluidigm SNPtype assays (Fluidigm®, San Francisco, USA) to build haplotypes and confirm the differences between Fedearroz 2000 and the seven new sources of resistance.
Results
Initial RHBV screening
Through mass evaluation, one genotype belonging to Group 2 was potentially RHBV resistant, and nine from Group 1 were statistically as resistant as the check, Fedearroz 2000 (data not shown).
Individual RHBV evaluation
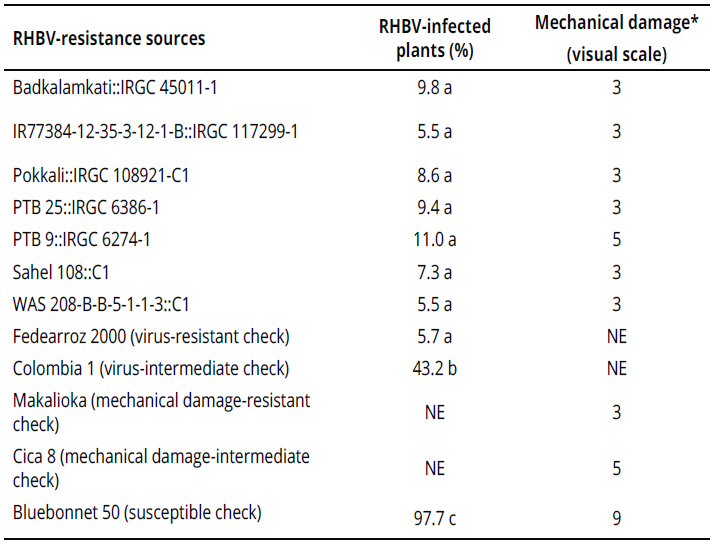
After individual confirmation of RHBV reaction, seven genotypes from Group 1 remained resistant under greenhouse conditions (Table 1). The remainder of the genotypes in both groups were susceptible (data not shown).
Field evaluation
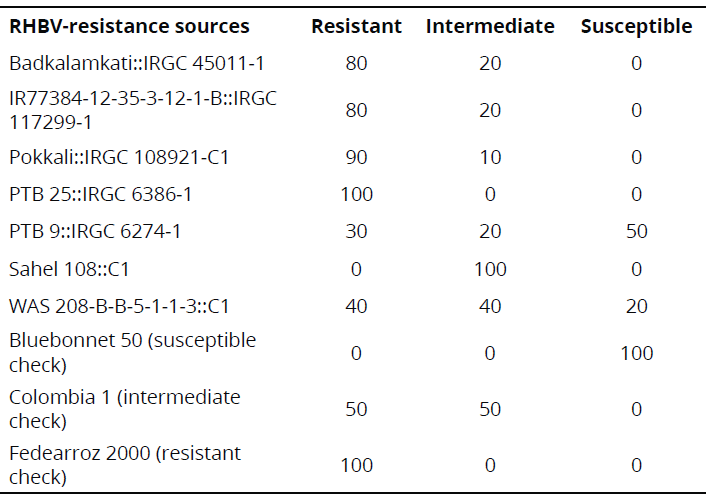
Under semi-controlled conditions in the field, four genotypes exhibited a high frequency of resistant rows compared with resistant check Fedearroz 2000: Badkalamkati, IR77384-12-35-3-12-1-B, Pokkali, and PTB 25. The genotypes PTB 9 and WAS 208-B-B-5-1-1-3 exhibited 50% and 20% of susceptible rows, respectively. And, the total number of rows of Sahel 108 was intermediate. None of the genotypes tested was as susceptible as Bluebonnet 50 (Table 2). Important traits for breeding purposes (Number of tillers, Number of panicles, 1000-grain weight, Number of total grains, Fertility, Plant height, Flowering time, and Cycle length) are shown in Tables 3 and 4.
Table 2 Frequency (%) of responses of indica genotypes exposed to RHBV under semi-controlled field conditions.
Reaction to insect damage
Six genotypes were as resistant to mechanical damage as Makalioka (Table 1). PTB 9 displayed an intermediate reaction, like that of Cica 8.
Molecular Analysis and Genetic Variability
The structure analysis revealed that the seven new sources of resistance belonged to three different subpopulations (Figure 1).
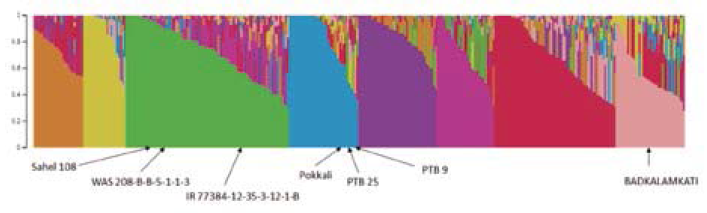
Figure 1 Population structure (k=8) of Group 1 (295 genotypes). New sources of resistance to RHBV are indicated within subpopulations.
Five genotypes representing the three subpopulations were submitted for WGS (Badkalamkati, IR77384-12-35-3-12-1-B, Pokkali, PTB 9, and WAS 208-B-B-5-1-1-3).
A total of 1514 SNPs was identified in the region between 2.81 Mbp and 3.80 Mbp of chromosome 4, which includes the RHBV-resistance QTL region of Fedearroz 2000 and Fedearroz 50 (Romero et al., 2014). According to the distance-based dendrogram (Figure 2), nine accessions analyzed were classified into four groups. Fedearroz 50 and Colombia 1 were close to Fedearroz 2000.
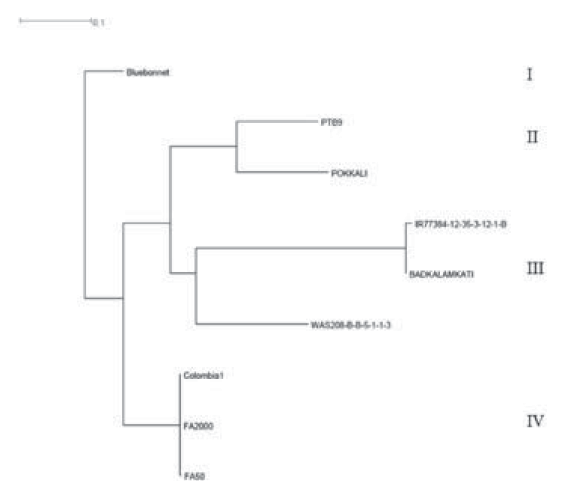
Figure 2 Neighbor joining dendrogram for the WGS accessions comparing the RHBV-resistance QTL region of Fedearroz 2000 (FA2000) and Fedearroz 50 (FA50).
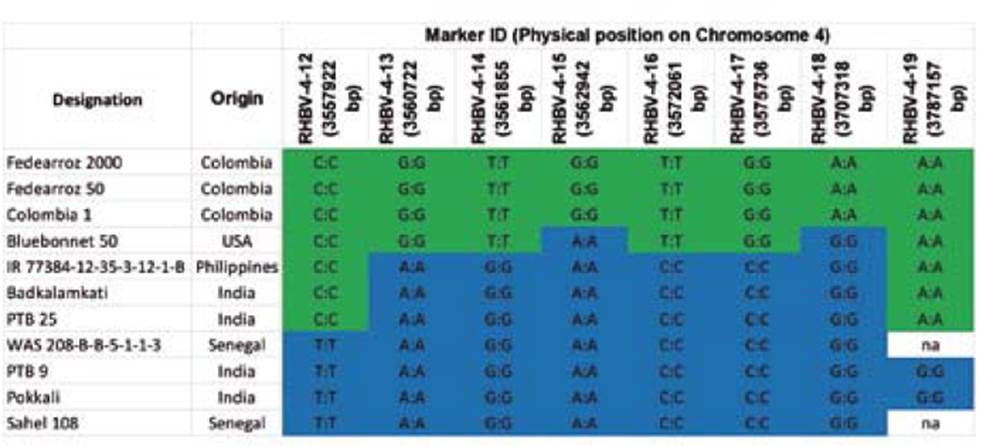
On the other hand, the new indica RHBV-resistance genotypes were classified into groups II and III, distant from Fedearroz 2000 in group IV. Furthermore, the eight SNPs developed as Fluidigm SNPtype assays allowed us to identify four haplotypes in the region on chromosome 4 (Table 5). Fedearroz 2000, Fedearroz 50, and Colombia 1 share the same haplotype, while the seven indica accessions were different.
Discussion
After Colombia 1, an unacceptable commercial variety, and Fedearroz 2000, the most RHBV-resistant variety widely sown in Colombian fields, there has been a limited quantity of sources with high RHBV resistance (Morales & Jennings, 2010) and no RHBV-resistant indica type has been identified. In crop management, this constitutes a latent risk, especially since the disease is currently spreading in tropical America. Moreover, past experience demonstrates that monocultures can suffer a breakdown of resistance because of genetic changes in the pathogen. The risk is even greater when plant health relies on a single genetic source of resistance (Kiyosawa, 1982).
Fedearroz 2000, Fedearroz 50, and Colombia 1 clustered together and were distant from the new indica accessions that show high levels of resistance to RHBV. The analysis of the genomic region surrounding and harboring the QTL suggested that the new sources of resistance are probably unique and different from the one in Fedearroz 2000 and Fedearroz 50. The origin of the resistance in these new sources needs to be clarified. It is still unclear whether it could be an allelic variation of the Fedearroz 2000 QTL or new genomic regions that are involved in resistance responses. Several biparental mapping populations are under development to distinguish between these possibilities.
The new RHBV-resistance sources identified in this research are from India, the Philippines, and Senegal, and belong to the indica type. Thus, it is feasible to use these potential sources in crosses targeting the tropics. In addition to RHBV resistance and tolerance of mechanical damage, the new sources are long-grained with intermediate to high amylose content as preferred by most Latin American consumers (Calingacion, Laborte, Nelson, Resurreccion, Concepcion, Daygon,. & Fitzgerald, 2014). Those are important advantages for rice breeding programs in the tropical zone. Future investigations will examine the putative antibiotic effects of the resistance to the insects observed in this study, the inheritance of the resistance and antibiotic traits, resistance gene discovery, and the design of molecular markers to accelerate plant breeding with these new resistance sources.